Fig. 3.1
Proportional reduction in major vascular events versus absolute low-density lipoprotein cholesterol (LDL-C) reduction. In a meta-analysis of 26 large trials of statin therapy, including a total of 170,000 patients, performed by the Cholesterol Treatment Trialists’ Collaboration, each 1-mmol/L (~38-mg/dL) decrease in LDL-C level decreased the first occurrence of any major coronary event, coronary revascularization, or stroke by 22 % (Data from Cholesterol Treatment Trialists’ (CTT) Collaboration [57])
Evidence for the Benefit of Lipid Therapy on Atherosclerosis
A large number of studies have shown the benefit of lipid therapy for the primary and secondary prevention of CVD events and atherosclerotic lesion progression [52, 53, 58–60]. The Scandinavian Simvastatin Survival Study (4S) [61] was a landmark study that was the first to show a significant reduction in all-cause mortality with lipid-lowering therapy. In 4,444 men and women with CHD, simvastatin 20–40 mg reduced all-cause mortality by 30 % (P = 0.0003) compared with placebo during the 5-year trial, mostly due to a 42 % reduction in CHD mortality [61]. In a 10-year follow-up evaluation, during which >80 % of patients in each treatment group received cholesterol-lowering drugs, all-cause mortality was still significantly reduced (by 15 %; P = 0.02) in patients randomized to receive simvastatin in the original trial, primarily owing to a 24 % reduction in CHD mortality [62]. In the Heart Protection Study (HPS) [63], in which about 20,000 patients with diabetes or occlusive arterial disease were randomized to receive simvastatin 40 mg or placebo, all-cause mortality was reduced by 13 % (P = 0.0003), mostly due to the 18 % reduction in coronary mortality (P = 0.0005). Simvastatin was beneficial in reducing the rate of vascular events regardless of the baseline LDL-C level; patients with a baseline LDL-C level of <100 mg/dL had a 22 % reduction in the rate of such events, which was comparable to the 24 % reduction in the trial overall [63].
Many clinical trials have also revealed the effect of lipid therapy on atherosclerotic lesions by means of imaging, including invasive modalities such as quantitative coronary angiography (QCA) and intravascular ultrasound (IVUS) and noninvasive modalities such as B-mode ultrasound and magnetic resonance imaging (MRI) [64–70]. In A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID), both IVUS [70] and QCA [72] were used in patients with CHD who were treated with rosuvastatin 40 mg for 2 years, and regression of coronary atherosclerosis was documented by both imaging modalities. An analysis that included ASTEROID and other clinical trials that used QCA to monitor the effects of statin therapy on coronary atherosclerotic lesions showed the benefit of more intensive lowering of LDL-C levels (Fig. 3.2) [72]. As LDL-C levels on treatment were further reduced with more efficacious statin therapy, CHD progression decreased; with even greater LDL-C reduction, CHD regression was observed. In the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) study, IVUS was used to evaluate atherosclerotic lesions in 654 patients who received either atorvastatin 80 mg or pravastatin 40 mg [73]. Of this group, 502 patients underwent IVUS at baseline and at 18 months, when the atheroma volume was increased by 2.7 % in the pravastatin group but no progression was observed in the atorvastatin group. In the Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin versus Atorvastatin (SATURN) [74], IVUS was used to compare the effects of different intensities of statin therapy on coronary atherosclerosis in 1,039 patients with CHD. At 2-year follow-up evaluation, rosuvastatin 40 mg resulted in significantly lower LDL-C levels than did atorvastatin 80 mg (63 mg/dL vs. 70 mg/dL, respectively); both treatments resulted in regression, as assessed by percent atheroma volume, but greater regression of the total atheroma volume was seen with rosuvastatin. In a smaller study in which IVUS was also used to compare intensities of lipid therapy, both rosuvastatin 20 mg and atorvastatin 40 mg resulted in regression of atherosclerotic lesions at 11-month follow-up examination, with no significant difference between the treatment groups; the investigators found that the LDL-C level at follow-up evaluation, not treatment, predicted disease progression [75].
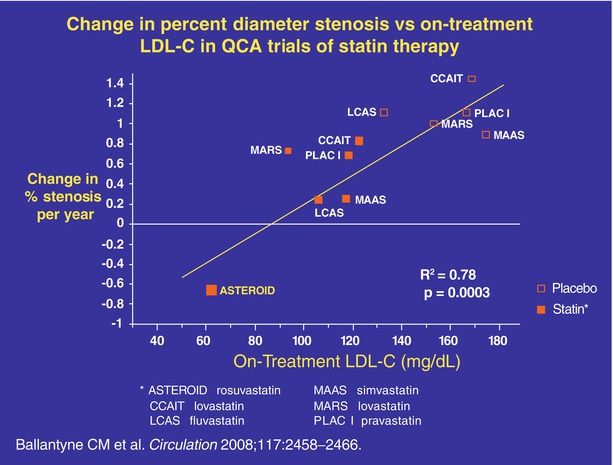

Fig. 3.2
Change in percent diameter stenosis vs on-treatment low-density lipoprotein cholesterol (LDL-C) in quantitative coronary angiography (QCA) trials of statin therapy. In a regression analysis based on data from trials of statin monotherapy with angiographic endpoints, coronary heart disease (CHD) progression decreased as LDL-C levels on treatment were further reduced with more efficacious statin therapy; with even greater LDL-C reduction, CHD regression was observed. Trials included were A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID) with rosuvastatin, Canadian Coronary Atherosclerosis Intervention Study (CCAIT) with lovastatin, Lipoprotein and Coronary Atherosclerosis Study (LCAS) with fluvastatin, Multicentre Anti Atheroma Study (MAAS) with simvastatin, Monitored Atherosclerosis Regression Study (MARS) with lovastatin, Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC I) with pravastatin, and Regression Growth Evaluation Statin Study (REGRESS) with pravastatin. Open boxes: placebo; closed boxes: statin (From Ballantyne et al. [72])
The Measuring Effects on Intima-Media Thickness: an Evaluation of Rosuvastatin (METEOR) study [76] evaluated the effect of lipid therapy on the change in carotid intima–media thickness (CIMT) as assessed by B-mode ultrasound in 984 patients with a low risk profile who were randomized to receive rosuvastatin 40 mg or placebo. The rosuvastatin group had a significantly slower rate of progression of CIMT compared with the placebo group. Compared with less intensive therapy, more intensive lipid therapy also resulted in regression of CIMT in other clinical trials [69, 77].
Atherosclerotic plaque volume has also been evaluated with MRI [78]. In the Outcome of Rosuvastatin Treatment on Carotid Artery Atheroma: a Magnetic Resonance Imaging Observation (ORION) trial [79], 43 patients were randomized to receive either low-dose (5-mg) or high-dose (40- or 80-mg) rosuvastatin, and 33 patients had MRI performed at baseline and at 2 years. Neither treatment group had a reduction in plaque volume; however, in all patients with plaques that had a lipid-rich necrotic core at baseline, the mean proportion of wall composed of lipid-rich necrotic core was reduced by 41 % (P = 0.005). In a randomized open-label study in which MRI was used to compare the effects of atorvastatin 20 mg and atorvastatin 5 mg on thoracic and abdominal plaques, thoracic plaques showed regression (15 % reduction in vessel wall area) with atorvastatin 20 mg but not with atorvastatin 5 mg (7 % increase in vessel wall area) [80].
Guidelines for the Management of Dyslipidemia
All current guidelines for the prevention of CVD, including those of the American College of Cardiology (ACC)/American Heart Association (AHA) [81], the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) [82], the Canadian Cardiovascular Society [83], and the International Atherosclerosis Society (IAS) [84], recommend assessment of the total CHD or cardiovascular risk because atherosclerotic CVD is the product of a number of risk factors. Many risk-assessment systems are available, such as the Framingham Risk Score [49], recommended in the Canadian and IAS guidelines, the Pooled Cohort Equation [85] used in the ACC/AHA guidelines, and Systemic Coronary Risk Evaluation (SCORE) [86] recommended in the ESC/EAS guidelines. The SCORE system estimates the 10-year risk of a first fatal atherosclerotic event—an MI, stroke, or other occlusive arterial disease event, including sudden cardiac death—whereas the Framingham system estimates only 10-year CHD risk but includes both fatal and nonfatal MI. The Pooled Cohort Equation estimates both 10-year and lifetime risks for atherosclerotic CVD, defined as coronary death, nonfatal MI, or fatal or nonfatal stroke. Based on SCORE estimates and CVD risk factors, the European guidelines assign total CVD risk categories, provide target LDL-C levels, and recommend treatment strategies for patients in each risk category (Tables 3.1 and 3.2). With the publication of the ACC/AHA guidelines, the US guidelines no longer recommend specific treatment targets but instead identify 4 risk groups within which varying intensities of statin therapy are recommended (Table 3.3).
Table 3.1
CVD risk categories and recommended LDL-C treatment targets in the European guidelines [82]
Risk category | Characteristics | LDL-C goal (mg/dL) |
|---|---|---|
Very high risk | Documented CVD | <70 |
Patients with type 2 diabetes, patients with type 1 diabetes with target organ | ||
damage (such as microalbuminuria) | ||
Patients with moderate to severe CKD (GFR <60 mL/min/1.73 m2) | ||
SCORE ≥10 % for 10 years | ||
High risk | Markedly elevated single risk factors such as familial dyslipidemias and severe hypertension | <100 |
SCORE ≥5 % and <10 % for 10 years | ||
Moderate risk | SCORE ≥1 % and <5 % for 10 years | <115 |
Low risk | SCORE <1 % | — |
Table 3.2
Intervention strategies as a function of total CVD risk and LDL-C level in the European guidelines [82]
Total CVD risk | LDL-C Level, mg/dL | ||||
|---|---|---|---|---|---|
<70 | 70 to <100 | 100 to <155 | 155 to <190 | ≥190 | |
SCORE <1 % | No intervention | No intervention | Lifestyle intervention | Lifestyle intervention | Lifestyle intervention; consider drug intervention if uncontrolled |
SCORE ≥1 % and <5 % | Lifestyle intervention | Lifestyle intervention | Lifestyle intervention; consider drug intervention if uncontrolled | Lifestyle intervention; consider drug intervention if uncontrolled | Lifestyle intervention; consider drug intervention if uncontrolled |
SCORE ≥5 % and <10 %, or high risk | Lifestyle intervention; consider drug intervention if uncontrolled | Lifestyle intervention, consider drug intervention if uncontrolled | Lifestyle intervention and immediate drug intervention | Lifestyle intervention and immediate drug intervention | Lifestyle intervention and immediate drug intervention |
SCORE ≥10 %, or very high risk | Lifestyle intervention; consider drug intervention if uncontrolled | Lifestyle intervention and immediate drug intervention | Lifestyle intervention and immediate drug intervention | Lifestyle intervention and immediate drug intervention | Lifestyle intervention and immediate drug intervention |
Table 3.3
Recommendations for cholesterol treatment to reduce CVD risk in ACC/AHA guidelines
Secondary prevention |
1. High-intensity statin therapya should be initiated or continued as first-line therapy in women and men ≤75 years of age who have clinical ASCVDb, unlesscontraindicated |
2. In individuals with clinical ASCVDb in whom high-intensity statin therapya would otherwise be used, when high-intensity statin therapy is contraindicated or when characteristics predisposing to statin-associated adverse effects are present, moderate-intensity statina should be used as the second option if tolerated |
3. In individuals with clinical ASCVD >75 years of age, it is reasonable to evaluate the potential for ASCVD riskreduction benefits and for adverse effects and drug–drug interactions and to consider patient preferences when initiating a moderate- or high-intensity statina. It is reasonable to continue statin therapy in those who are tolerating it |
Primary prevention in individuals ≥21 years of age with LDL-C ≥190 mg/dL |
1. Individuals with LDL-C ≥190 mg/dL or triglycerides ≥500 mg/dL should be evaluated for secondary causes of hyperlipidemia |
2. Adults ≥21 years of age with primary LDL-C ≥190 mg/dL should be treated with statin therapy (10-year ASCVD risk estimation is not required): |
Use high-intensity statin therapya unless contraindicated |
For individuals unable to tolerate high-intensity statin therapy, use the maximum tolerated statin intensity |
3. For individuals ≥21 years of age with an untreated primary LDL-C ≥190 mg/dL, it is reasonable to intensify statin therapy to achieve ≥50 % LDL-C reduction |
4. For individuals ≥21 years of age with an untreated primary LDL-C ≥190 mg/dL, after the maximum intensity of statin therapy has been achieved, addition of a nonstatin drug may be considered to further lower LDL-C. Evaluate the potential for ASCVD risk-reduction benefits, adverse effects, and drug–drug interactions, and consider patient preferences |
Primary prevention in individuals with diabetes and LDL-C 70–189 mg/dL |
1. Moderate-intensity statin therapya should be initiated or continued for adults 40–75 years of age with diabetes |
2. High-intensity statin therapya is reasonable for adults 40–75 years of age with diabetes with a ≥7.5 % estimated 10-year ASCVD risk unless contraindicated |
3. In adults with diabetes, who are <40 years of age or >75 years of age, or with LDL-C <70 mg/dL it is reasonable to evaluate the potential for ASCVD benefits and for adverse effects and drug–drug interactions and to consider patient preferences when deciding to initiate, continue, or intensify statin therapy |
Primary prevention in individuals without diabetes and with LDL-C 70–189 mg/dL |
1. The Pooled Cohort Equations should be used to estimate 10-year ASCVDc risk for individuals with LDL-C 70–189 mg/dL without clinical ASCVDb to guide initiation of statin therapy for the primary prevention of ASCVD |
2. Adults 40–75 years of age with LDL-C 70–189 mg/dL, without clinical ASCVDb or diabetes, and with an estimated 10-year ASCVDc risk 7.5 % should be treated with moderate- to high-intensity statin therapya |
3. It is reasonable to offer treatment with a moderate-intensity statina to adults 40–75 years of age, with LDL-C 70–189 mg/dL, without clinical ASCVDb or diabetes, and with an estimated 10-year ASCVD riskc of 5 % to <7.5 % |
4. Before initiation of statin therapy for the primary prevention of ASCVD in adults with LDL-C 70–189 mg/dL without clinical ASCVDb or diabetes, it is reasonable for clinicians and patients to engage in a discussion that considers the potential for ASCVD risk-reduction benefits and for adverse effects and drug–drug interactions, as well as patient preferences for treatment |
5. In adults with LDL-C <190 mg/dL who are not otherwise identified in a statin benefit group, or for whom after quantitative risk assessment a risk-based treatment decision is uncertain, additional factorsd may be considered to inform treatment decision making. In these individuals, statin therapy for primary prevention may be considered after evaluation of the potential for ASCVD risk-reduction benefits, adverse effects, and drug–drug interactions and consider patient preferences |
In most major guidelines for dyslipidemia management, LDL-C remains the primary target of therapy. Apo B can be substituted for LDL-C; recommended treatment targets for apo B are <80 mg/dL in individuals with a very high CVD risk; <100 mg/dL in individuals with a high CVD risk as defined by both the ESC/EAS guidelines and the American Diabetes Association/American College of Cardiology Foundation consensus statement [82, 87]; and ≤80 mg/dL in individuals with an intermediate or a high CVD risk as defined by the Canadian guidelines [83]. Non-HDL-C (total cholesterol – HDL-C) can also be a treatment target, with goals ~30 mg/dL higher than the corresponding LDL-C target [82–84]. Lifestyle modification is recommended as an initial therapy for all patients who have an increased CVD risk, and this approach should be combined with drug therapy in patients in higher-risk categories. In the major guidelines, statin therapy remains the first-line choice for the pharmacologic treatment of hypercholesterolemia. In patients whose treatment goals are not achieved with statins, other medications may be added.
Lipid-Lowering Agents
Statin, fibric acid derivatives, nicotinic acid (niacin), cholesterol absorption inhibitors, bile acid sequestrants, and omega-3 fatty acids are commonly used to treat lipid disorders. These agents have different mechanisms of action and, therefore, different effects on cholesterol and triglyceride levels.
Statins
Statins are the most commonly used lipid-lowering medications. They work by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which is the rate-limiting enzyme in cholesterol synthesis. This inhibition leads to a decrease in hepatic intracellular cholesterol levels, resulting in increased uptake of LDL through upregulation of hepatic LDL receptors. Seven statins are approved for use in the United States and have different LDL-C–lowering efficacies (Table 3.4). Statins with a short half-life are best taken in the evening because HMG-CoA reductase activity reaches its maximum level at night, but this is not true of atorvastatin or rosuvastatin, both of which have much longer half-lives. The peak effect of statin therapy appears in 2–4 weeks, and lipid levels should be measured at least 6 weeks after initiation of therapy [88]. Statins are generally safe medications with a low incidence of adverse effects, mainly involving the muscles or liver, and these effects are typically resolved either by decreasing the dose or by switching to a different statin [88, 89]. Recent reports have described an increased risk of developing diabetes mellitus with statin therapy, and this risk depends on both the particular statin and the dose [90].
High-intensity (~ ≥50 % LDL-C reduction) | Moderate-intensity (~30 % to <50 % LDL-C reduction) | Low-intensity (~ <30 % LDL-C reduction) |
|---|---|---|
Atorvastatin 40–80 mg/day | Atorvastatin 10–20 mg/day | Fluvastatin 20–40 mg/day |
Rosuvastatin 20–40 mg/day | Fluvastatin 40 mg BID | Lovastatin 20 mg/day |
Fluvastatin XL 80 mg/day | Pitavastatin 1 mg/day | |
Lovastatin 40 mg/day | Pravastatin 10–20 mg/day | |
Pitavastatin 2–4 mg/day | Simvastatin 10 mg/day | |
Pravastatin 40–80 mg/day | ||
Rosuvastatin 5–10 mg/day | ||
Simvastatin 20–40 mg/day |
Statins are widely used for the treatment of hypercholesterolemia and mixed hyperlipidemia. These agents are highly effective in lowering LDL-C levels (see Table 3.4) and can also lower triglyceride levels and increase HDL-C levels to a lesser degree. Statins have been proved to be beneficial in both primary and secondary prevention of atherosclerotic CVD events. The largest and most recent study to address the effect of lipid therapy for primary prevention was Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) [58]. This randomized, double-blind clinical trial included 17,802 patients without CHD or diabetes who had an LDL-C level of <130 mg/dL and a high-sensitivity C-reactive protein (hs-CRP) level of ≥2 mg/L. Patients were randomized to receive rosuvastatin 20 mg daily or placebo. The planned 5-year trial was stopped at a median follow-up interval of 1.9 years because of a clear benefit with rosuvastatin treatment, which reduced LDL-C and hs-CRP levels by 50 and 37 %, respectively, and resulted in a 44 % reduction of the composite primary endpoint (nonfatal MI, nonfatal stroke, hospitalization for unstable angina, CVD death, and revascularization) and a 20 % reduction in all-cause mortality [58]. Figure 3.3 shows the cumulative incidence of cardiovascular events according to study group in the JUPITER trial. Figure 3.4 shows the relationship of the proportional reduction in cardiovascular event rate and mean LDL-C difference between treatment groups in published statin trials [58]. A Cochrane review of statin use for primary prevention in 48,060 participants from 13 clinical trials showed a 14 % reduction in all-cause mortality and a 27 % reduction in fatal and nonfatal CHD events [91]. In a meta-analysis of data from 90,056 individuals in 14 statin trials, which included both primary and secondary prevention studies, each 1-mmol/L (38.7-mg/dL) reduction in the LDL-C level was associated with a 12 % reduction in all-cause mortality and a 19 % reduction in coronary mortality [92]. Many large clinical trials have established the benefit of statins in secondary prevention (see Evidence for the Benefit of Lipid Therapy on Atherosclerosis, above), including several trials that compared the clinical benefits of more-intensive and less-intensive statin therapy, either with different statins or with different doses of the same statin. The Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE IT–TIMI-22) study [93] showed a benefit of statin therapy in patients with acute coronary syndrome as early as 30 days after the event and showed a benefit with more-intensive LDL-C lowering. In this study, 4,162 patients were randomized to receive pravastatin 40 mg or atorvastatin 80 mg. After 2 years of follow-up observation, patients treated with atorvastatin had a 16 % reduction of primary endpoints (death, MI, documented unstable angina requiring hospitalization, coronary revascularization, or stroke) compared with the pravastatin group. In the Treating to New Targets (TNT) study [59], 10,001 patients with stable CHD were randomized to receive either atorvastatin 80 mg or atorvastatin 10 mg. The primary endpoint, occurrence of a first major cardiovascular event (CHD death, nonfatal non–procedure-related MI, resuscitated cardiac arrest, or fatal or nonfatal stroke) was significantly reduced by 22 % in the group receiving atorvastatin 80 mg at a median follow-up interval of 4.9 years. The total mortality rate was not significantly different in the two groups [59]. The Incremental Decrease in Endpoints through Aggressive Lipid Lowering (IDEAL) study [60] compared the effects of atorvastatin 80 mg and simvastatin 40 mg on major coronary events (coronary death, confirmed nonfatal acute MI, or resuscitated cardiac arrest) in 8,888 patients with a previous MI and found an 11 % reduction with atorvastatin 80 mg at a median follow-up interval of 4.8 years. As in the TNT study, the total mortality rate was not different between treatment groups in IDEAL [60]. In the Study of the Effectiveness of Additional Reduction of Cholesterol and Homocysteine (SEARCH) [94], simvastatin 80 mg and simvastatin 20 mg were compared in 12,064 patients who had a previous MI. At a mean follow-up interval of 6.7 years, the primary endpoint of major vascular events (coronary death, MI, stroke, or arterial revascularization) was not significantly different in the two treatment groups. However, the incidence of confirmed myopathy was significantly increased by 27 % in patients randomized to receive simvastatin 80 mg (53 [1 %] patients, vs. 2 [0.03 %] patients randomized to receive simvastatin 20 mg), including 7 patients (0.1 %) receiving simvastatin 80 mg who had rhabdomyolysis, which was not reported in the 20-mg group.
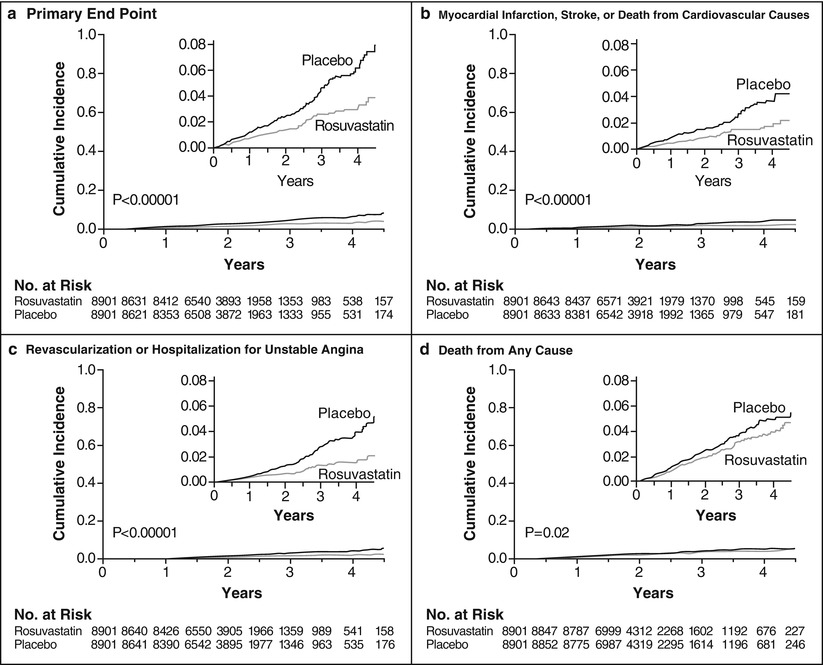
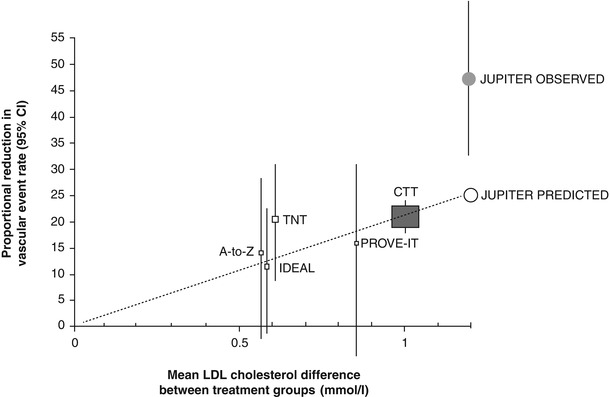

Fig. 3.3
Cumulative incidence of cardiovascular events according to study group. Panel (a) shows the cumulative incidence of the primary end points (nonfatal myocardial infarction, nonfatal stroke, arterial revascularization, hospitalization for unstable angina, or confirmed death from cardiovascular causes). The hazard ratio for rosuvastatin, as compared with placebo, was 0.56 (95 % confidence interval [CI], 0.46–0.69; P < 0.00001). Panel (b) shows the cumulative incidence of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes, for which the hazard ratio in the rosuvastatin group was 0.53 (95 % CI, 0.40–0.69; P < 0.00001). Panel (c) shows the cumulative incidence of arterial revascularization or hospitalization for unstable angina, for which the hazard ratio in the rosuvastatin group was 0.53 (95 % CI, 0.40–0.70; P < 0.00001). Panel (d) shows the cumulative incidence of death from any cause, for which the hazard ratio in the rosuvastatin group was 0.80 (95 % CI, 0.67–0.97; P = 0.02). I n each panel, the inset shows the same data on an enlarged y axis and on a condensed x axis (From Ridker et al. [58]. Copyright ©2008 Massachusetts Medical Society. Reprinted with permission)

Fig. 3.4
Relationship of the proportional reduction in cardiovascular event rate and mean low-density lipoprotein (LDL) cholesterol difference between treatment groups in published statin trials. Gray square represents summary data from randomized trials of statin therapy versus placebo as summarized by the Cholesterol Treatment Trialists’ (CTT) Collaboration, and solid squares represent results of individual trials comparing different intensities of statin therapy. Open circle represents projected benefit of rosuvastatin and closed circle represents observed benefit in the JUPITER trial. Vertical lines are 95 % confidence intervals. PROVE IT Pravastatin or Atorvastatin Evaluation and Infection Therapy, IDEAL Incremental Decrease in End Points through Aggressive Lipid-lowering, TNT Treating to New Targets, A-to-Z Aggrastat-to-Zocor (From Ridker et al. [58]. Copyright ©2008 Massachusetts Medical Society. Reprinted with permission. Adapted by Ridker et al. from SEARCH Study Collaborative Group. [145])
Statin treatment has also been shown to provide a benefit in patients with chronic kidney disease (CKD) [95, 96]. A meta-analysis of data from 80 clinical trials in which a total of 51,099 patients with CKD received either a statin or placebo showed reductions in all-cause mortality (19 %), CVD mortality (22 %), and CVD events (24 %) with statin therapy in CKD patients not undergoing hemodialysis [97].
Fibric Acid Derivatives
Fibrates are effective in lowering triglyceride levels and also increasing HDL-C levels to a lesser degree. Fibrates are peroxisome proliferator–activated receptor–α (PPAR-α) agonists [98]. They decrease very low density lipoprotein (VLDL) secretion from the liver by reducing hepatic lipogenesis. They also increase lipoprotein lipase activity and decrease apo C-III concentration, which enhances degradation of VLDL. Fibrates increase the size of LDL particles, and this might explain the increase in LDL-C after the initiation of fibrate therapy in some individuals [99, 100]. Fenofibrate may be considered concomitantly with a low- or moderate-intensity statin only if the benefits from ASCVD risk reduction or triglyceride lowering when triglycerides are >=500 mg/dL are judged to outweigh the potential risk for adverse effects. Although, in general, fibrates are relatively safe, muscle pain, myositis, and rhabdomyolysis may occur. Combining statins and fibrates increases the risk; although the combination is not contraindicated, it necessitates cautious patient selection and close monitoring [88]. Clinical trials suggest that fewer muscle side effects occur with the combination of statin and fenofibrate than with statin and gemfibrozil [101]. The gemfibrozil dose should be decreased by 50 % (to 600 mg daily) if the glomerular filtration rate (GFR) is <60 mL/min/1.73 m2. The fenofibrate dose should be decreased by 50 % if the GFR is between 60 and 90 mL/min/1.73 m2 and by 75 % if the GFR is between 15 and 59 mL/min/1.73 m2. Fibrates are contraindicated if the GFR is less than 15 mL/min/1.73 m2 [102].
Although statins are the primary agents in the treatment of hypercholesterolemia, patients with high triglyceride or low HDL-C levels may benefit from the addition of fibrates [101]. A meta-analysis showed that fibrates can reduce CVD or coronary events but do not decrease mortality [103]. In the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT), gemfibrozil therapy in men with CHD and a low HDL-C level resulted in fewer CHD events than did placebo [104]. However, these trials were performed before statins were the standard of care and, thus, were not designed to examine whether fibrates provide additional benefit in reducing CHD risk in patients on statin therapy.
In contrast to these findings, fenofibrate trials such as the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study [105] and the lipid arm of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study [106] did not provide evidence that adding fenofibrate to statin therapy produced a benefit in reducing CVD events. However, in a subset analysis of ACCORD, patients with high triglyceride and low HDL-C levels showed a trend toward CVD event reduction with fenofibrate [106]. The Evaluation of Fenofibric Acid on Carotid Intima-Media Thickness in Patients with Type IIb Dyslipidemia with Residual Risk in Addition to Atorvastatin Therapy (FIRST) Trial also failed to show a benefit on CIMT with the use of fenofibric acid in patients with low HDL-C and high triglyceride levels who were already treated with a statin [107]. According to the ACC/AHA guidelines, “Fenofibrate may be considered concomitantly with a low- or moderate-intensity statin only if the benefits from ASCVD risk reduction or triglyceride lowering when triglycerides are ≥500 mg/dL are judged to outweigh the potential risk for adverse effects [81].”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


