CHAPTER 57 Medical Management of Acute Coronary Syndromes
The term acute coronary syndromes (ACS) refers to the spectrum of conditions compatible with acute myocardial ischemia, from unstable angina to acute myocardial infarction (MI). These disorders are a major cause of morbidity and mortality around the world. In the United States alone, more than 650,000 people will have a new ACS event every year, and another 450,000 will have a repeated occurence.1 Just under half of these people will die of their ACS event. ACS also entails major financial costs; in 1998 alone, Medicare paid $10.6 billion in hospital costs for ACS.1
The adverse clinical and financial consequences of ACS may be exacerbated by a lack of adherence to practice guideline recommendations. Since 1980, the American College of Cardiology (ACC) and the American Heart Association (AHA) jointly have published guidelines for the treatment of various cardiovascular diseases. In 2002, the ACC/AHA issued revised guidelines for the management of patients with unstable angina or non–ST-segment elevation acute MI (NSTEMI),2 the two conditions that together make up non–ST-segment elevation ACS (NSTE ACS). For ST-segment acute MI (STEMI, the remaining disorder of ACS), the guidelines were last updated in 2008.3 Whenever possible, the recommendations given in this chapter reflect these practice guidelines, which represent a consensus of relevant professional societies and are meant to assist physicians in making the most appropriate, evidence-based decisions about the management of their patients in specific circumstances.
PATHOPHYSIOLOGY OF ACUTE CORONARY SYNDROMES
Inflammatory Response to Injury
Various risk factors cause inflammatory changes in the circulation and vessel wall, including excess low-density lipoprotein (LDL) cholesterol and oxidized LDL cholesterol, other sources of oxidative stress, cigarette smoking, hypertension, and diabetes mellitus. Recently, immune responses and viral infections have also been implicated, although these findings remain controversial.1,2,4,5 In a sense, it can be argued that atherosclerosis is the common response to combinations of these insults. Most recently, the concept that vascular progenitor cells from the marrow play a role in vessel repair has garnered intense interest. As this stem cell function becomes less effective with aging, the inability to repair vascular damage after inflammation may prove a critical factor in the relationship between aging and atherosclerosis.
For patients who undergo percutaneous coronary intervention (PCI), the procedure itself can traumatize the vessel wall, but the response to this mechanical injury is more fibrotic. Therefore, ACS rarely occurs after PCI by plaque fissuring at the site of the PCI, except in the immediate peri-procedural period. In contrast, the lumen of saphenous vein grafts is prone to lipid-rich atheroma and superimposed diffuse thrombosis so that over time, vein grafts become sources of unstable atheromatous mass.
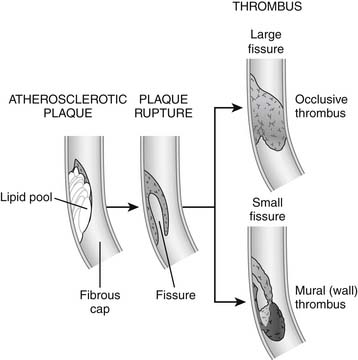
Plaque Rupture
The most dangerous kind of plaque is a nonobstructive, lipid-rich plaque with a thin fibrous cap, often called vulnerable plaque, which is most prone to rupture. There may be gender differences in plaque behavior; women appear to be more prone than men to development of fissuring as opposed to rupture, with its correspondingly higher risk of nonocclusive thrombus (Fig. 57-1).
The degree of damage depends on the distribution of myocardium supplied by the affected artery; the degree of obstruction by the clot; the amount of small vessel obstructed by inflammation, thrombotic emboli, and vasoconstriction; and the extent of collateral flow. Even if the thrombus resulting from the original rupture does not obstruct the artery, smaller clots generated by the process (microemboli) can travel downstream to smaller arteries, occluding the microvasculature and causing myocardial necrosis.6,7
Angiographic and autopsy studies have shown that unstable angina and NSTEMI often reflect intermittent occlusion of coronary arteries with spontaneous recovery of blood flow to affected tissue (reperfusion).8 Thrombotic occlusion is more persistent in STEMI because plaque damage is usually more severe or because collateral flow is absent.
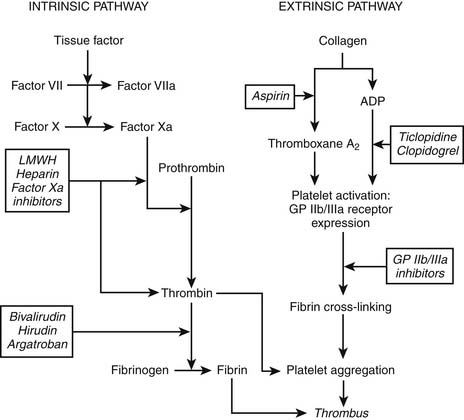
Role of Platelets in ACS
Plaque rupture leads to vessel wall damage, which in turn stimulates the initiation of two pathways toward coagulation (Fig. 57-2). The intrinsic pathway of the coagulation cascade ultimately results in the production of thrombin. Thrombin is an enzyme that splits fibrinogen into fibrin monomers, which, when cross-linked, stabilize a platelet-rich thrombus by forming a net. Thrombin also amplifies the coagulation process by stimulating further thrombin generation. In addition, fibrin-bound thrombin activates (1) factor XIII, a plasma enzyme that stabilizes the linked fibrin threads, and (2) carboxypeptidase B, another enzyme that reduces the body’s intrinsic fibrinolytic process.
DIAGNOSIS AND EPIDEMIOLOGY OF ACUTE CORONARY SYNDROME SUBTYPES
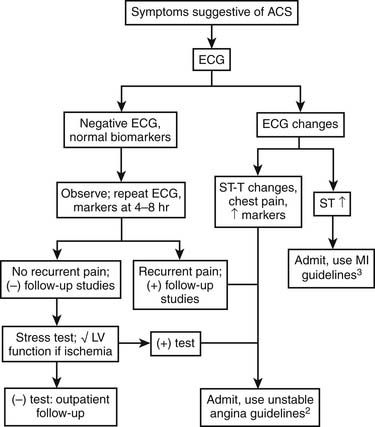
Patients with possible ACS are categorized by the presence or absence of persistent ST-segment elevation on electrocardiography and by the level of various biomarkers in their circulation (Fig. 57-3). This nomenclature is critical, because patients with persistent ST-segment elevation require lifesaving rapid reperfusion therapy, whereas no benefit of acute reperfusion therapy has been found in patients without ST-segment elevation. Patients with ACS but no persistent ST-segment elevation (and those with other electrocardiographic abnormalities) are retrospectively categorized as having unstable angina or NSTEMI, with or without Q waves, once the results of biomarker tests are available. This terminology is more descriptive than previous classifications in that it reflects the need for clinicians to make immediate therapeutic decisions once the medical history and electrocardiogram have been obtained. The epidemiology of ACS is changing, with more NSTE ACS and fewer STEMI cases being observed. These changes in the ACS population seem to reflect the aging of society (older patients have a higher proportion of NSTE ACS than STEMI) coupled with widespread use of effective secondary prevention measures that preclude the complete vessel occlusion required to produce STEMI. The adoption of troponin measurement as a standard of care has further expanded the population of NSTEMI patients who previously would have been classified as having unstable angina.
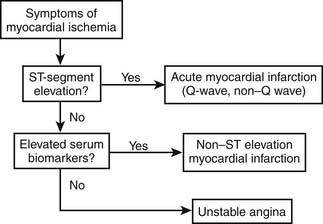
Figure 57–3 Categorization of patients with possible acute coronary syndromes.
(Modified from American Heart Association. Heart disease and stroke statistics—2003 update. Dallas: American Heart Association; 2003.)
Unstable coronary syndromes may be classified according to the Canadian Cardiovascular Society classification, which grades symptoms from class I to class IV (Table 57-1).9 This classification of angina is useful both prognostically and therapeutically. Patients with advanced Canadian Cardiovascular Society anginal symptoms have a higher risk of death from MI than do patients with more stable symptoms. There also is some suggestion that more potent therapies may be best suited to patients with the most advanced disease.
Table 57–1 Canadian Cardiovascular Society Classification of Anginal Symptoms9
| Class | Description |
|---|---|
| I | No angina with ordinary activity; pain with strenuous exertion |
| II | Slight limitation of ordinary activity |
| III | Marked limitations of ordinary activity |
| IV | Pain with any physical activity; symptoms may occur at rest |
INITIAL RISK STRATIFICATION
Information from history, physical examination, electrocardiography, and biomarker tests is used to assign patients to one of four categories: noncardiac disorders, chronic stable angina, possible ACS, and definite ACS. This chapter is focused on the last two groups (Fig. 57-4).
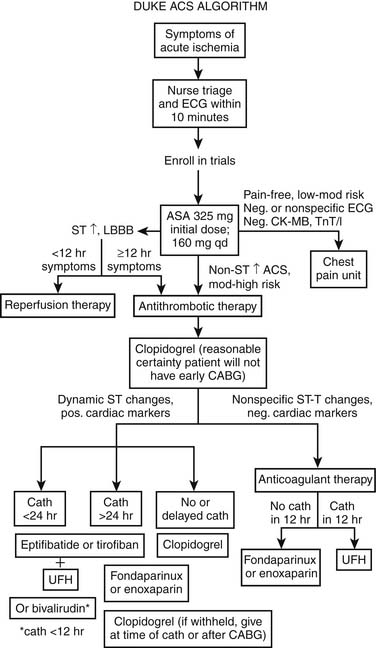
Figure 57-5 shows an algorithm by which patients with symptoms of cardiac ischemia are treated at Duke University Medical Center.1 Patients with possible ACS are candidates for additional observation in a specialized facility (such as a chest pain unit).10 Patients with definite ACS, as noted, are managed primarily according to the electrocardiographic pattern; those with ST-segment elevation are possible candidates for immediate reperfusion therapy and are managed according to American College of Cardiology and American Heart Association (ACC/AHA) practice guidelines for acute STEMI3; those without ST-segment elevation either undergo further observation or are admitted.2 Patients with low-risk ACS and no transient ST-segment depression (≥0.05 mV) or T-wave inversions (≥0.2 mV), no positive cardiac markers or hemodynamic abnormalities, and no positive stress test result may be discharged and seen as outpatients.1 This algorithm pertains to Duke University Medical Center only and should not be construed as presenting the definitive standard of care or containing diagnostic or therapeutic recommendations.
The prognosis of patients with NSTE ACS with positive markers or electrocardiographic abnormalities, contrary to the conventional wisdom, actually is worse than that of patients with acute STEMI; those with ST-segment depression, for example, have a mortality rate at 6 months of 8.9% compared with 6.8% for those with ST-segment elevation.11 The risks of death and of death or MI at 30 days can be calculated for the general ACS population without STEMI according to a predictive model developed from the large Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) trial database (Fig. 57-6).12 Perhaps not surprisingly, age, electrocardiographic pattern, and heart failure account for most of the risk. What is particularly useful about this model, however, is that use of these variables to determine early clinical risk also may allow us to predict which patients may derive the most benefit from more aggressive treatment.
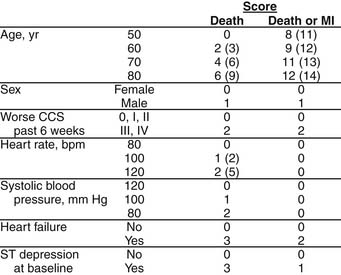
(Reprinted with permission from Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcomes in patients with acute coronary syndromes without persistent ST-segment elevation: results from an international trial of 9461 patients12.)
For acute MI, the risk of death at 30 days was 7.0% in the Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries (GUSTO-I) trial, the largest trial of fibrinolysis in MI to date.13 (The rate had increased to 9.6% by 1 year.14) Among patients who died, the expected risk factors predominated; the most powerful of these were age, heart rate, hemodynamics, prior MI, and location of current MI. Figure 57-7 shows a nomogram for predicting 1-year mortality after MI among 30-day survivors, with use of the three most predictive baseline clinical, electrocardiographic, and in-hospital variables.14 The only unexpected finding regarding risk factors predictive of mortality at 1 year versus 30 days was the poor long-term prognosis of black patients.14 After adjustment for other prognostic factors, including revascularization, black patients still had more than twice the risk of white patients for late mortality in GUSTO-I. Differential access to or use of secondary prevention measures may explain some of this disparity.
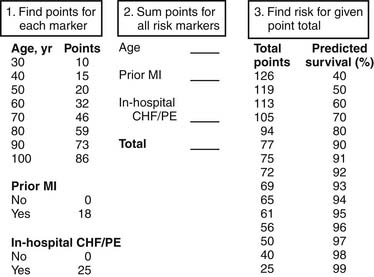
(Reprinted with permission from Califf RM, Pieper KS, Lee KL, et al. Prediction of 1-year survival after thrombolysis for acute myocardial infarction in the GUSTO-I trial14.)
Cardiac biomarkers, especially the troponins, play an important role in risk stratification of patients with ACS, particularly those without ST-segment elevation. In the Global Use of Strategies To Open Occluded Coronary Arteries (GUSTO-IIa), for example, a positive troponin T result at baseline was highly predictive of 1-year mortality.15 At 1 year, mortality among patients who were troponin positive at arrival was 14% compared with 5% among patients who were initially troponin negative. The Thrombolysis In Myocardial Infarction (TIMI) group has shown a strong association between baseline troponin I level and 42-day mortality in NSTE ACS.16 Moreover, a positive baseline troponin T level can predict 1-year mortality in ACS, even after adjustment for other baseline clinical and electrocardiographic characteristics.17
Troponin levels also add predictive information even in the presence of STEMI.15 Among patients with MI in the GUSTO-IIa trial, those with a positive baseline troponin T level had higher rates of death at 30 days (13% versus 4.7%), in-hospital repeated MI (91% versus 81%), and bypass surgery (16% versus 14%) compared with troponin-negative patients with STEMI.
Positive biomarkers predict not only which patients are at higher risk for adverse outcomes but also those who might benefit most from aggressive treatment. Lindahl and colleagues reported a greater benefit of dalteparin among baseline troponin T–positive patients (versus troponin T–negative) in the Fragmin In Unstable Coronary Artery Disease (FRISC) trial.18 Other trials have shown preferential effects of GP IIb/IIIa inhibitors among patients with NSTE ACS who are troponin positive at baseline.17–20 Finally, the benefits of an invasive versus a conservative mode of therapy may be greater for patients who are troponin positive on arrival.21 Troponins appear to be a powerful tool for risk stratification and selection of aggressive medical and interventional therapies.
A biomarker of more recent interest is C-reactive protein. Although it is generally recognized as a marker of systemic inflammation and has recently been noted as a new risk marker for chronic coronary artery disease, the role of C-reactive protein in ACS has been less prominently discussed. In the recent GUSTO-IV ACS trial, elevated C-reactive protein level was a potent predictor of recurrent vascular events.21
Even more recently, several groups have shown the prognostic value of measurement of atrial natriuretic peptide and brain natriuretic peptide levels in patients with NSTE ACS.22,23 This marker reflects atrial stretch, most often due to heart failure, in this population. Patients with elevated atrial natriuretic peptide and brain natriuretic peptide levels had a significantly increased risk of death during follow-up.
An approach that has gained in favor as data have accumulated is the “multimarker” strategy, initially introduced and recently confirmed in a randomized trial by Newby and colleagues.24 This approach, which refers to the simultaneous use of troponin, creatine kinase (CK)–MB, and myoglobin, might be further enhanced by including C-reactive protein and brain natriuretic peptide in the mix of markers. In the future, arrays of protein biomarkers will likely be used to stratify prognosis, to determine preferred treatment alternatives, and to examine the biological impact of treatment for both research and clinical care.
MEDICAL VERSUS CATHETERIZATION-BASED STRATEGIES
Once a patient’s risk has been estimated, the choices for management are split into two broad categories: medical management and catheter-based or surgical strategies. Table 57-2 details the definitive recommendations for early invasive approaches and procedures from the NSTE ACS and STEMI guidelines.2,3 The bottom line for both STEMI and NSTE ACS is that for most patients with medium or high risk, catheterization is the critical component of treatment, which also should include medical therapy. Thus, medical therapy is seen not as an alternative to invasive evaluation and management but rather as an adjunctive but crucial component of the invasive strategy.
Table 57–2 Guideline Class IA Recommendations for Early Invasive Strategy (versus Medical Therapy Only) for Patients with NSTE ACS2 and STEMI3
| Characteristics | Treatment |
|---|---|
| NSTE ACS | |
Angina that recurs; occurs at rest; occurs with heart failure symptoms, S3 gallop, pulmonary edema, worsening rales, or new or worsening mitral regurgitation; or occurs with low-level activity despite intensive anti-ischemic therapy | Early angiography, possible intervention |
| Left main disease,∗ candidate for bypass | Bypass |
| Three-vessel disease with ejection fraction <50% | Bypass |
| Multivessel disease including proximal left anterior descending coronary artery, ejection fraction <50%, or untreated diabetes | Bypass |
| Multivessel disease with ejection fraction >50%, no diabetes† | PCI |
| STEMI | |
| If able to be performed <12 hours after symptom onset or >12 hours if symptoms persist, if able to be performed <90 minutes at experienced centers‡ | PCI |
| Persistent or recurrent symptomatic ischemia, spontaneous or induced, with or without electrocardiographic changes | PCI |
| Cardiogenic shock, severe pulmonary congestion, continuing hypotension, if able to be performed <18 hours after onset | PCI |
| Failed angioplasty with persistent pain or hemodynamic instability; suitable anatomy | Bypass |
| Persistent, recurrent, or refractory ischemia; suitable anatomy; not a candidate for PCI | Bypass |
| Surgical repair of ventricular septal defect of mitral valve insufficiency | Bypass |
† Class/level of evidence I/A if severe angina persists despite medical therapy.
‡ Operators performing more than 75 percutaneous coronary intervention (PCI) procedures per year; centers performing more than 200 procedures per year that have cardiac-surgery capability.
Modified from the ACC/AHA guidelines.2,3
In general, for patients with NSTE ACS, medical management should be reserved only for those who are low risk, as evidenced by lack of electrocardiographic changes, hemodynamic abnormalities, or cardiac markers, and for patients in whom procedures are contraindicated. The FRISC II25–26 and Treat Angina with Aggrastat and Determine the Cost of Therapy with Invasive or Conservative Strategies (TACTICS)–TIMI 18 trials27 both have shown the superiority of an early invasive strategy versus a more conservative one. In FRISC II, however, randomization occurred after approximately 5 days of treatment with dalteparin. Thus, although the catheterization-based strategy appeared to benefit patients and dalteparin was beneficial during the waiting period, this approach is unlikely to be adopted because of the cost of prolonged observation. In the TACTICS–TIMI 18 trial, patients in the invasive arm underwent angiography much more rapidly than in FRISC II (median, 24 hours) with revascularization to follow. As with FRISC II, the invasive strategy was associated with significantly improved clinical outcomes. Also, in agreement with the troponin substudy of FRISC II,25 the troponin-positive patients in TACTICS–TIMI 18 derived particular benefit from more aggressive management, showing an absolute 10% reduction in the primary endpoint (compared with an absolute 3.5% reduction for the trial population as a whole). Most recently, the third British Randomised Intervention Trial of Unstable Angina (RITA 3) also showed a benefit of early, aggressive intervention in this population.28
Recent trials in chronic stable coronary artery disease have shown only a modest symptomatic benefit for revascularization compared with modern medical therapy,29,30 raising the question of whether the early interventional approach should be reassessed. Several major trials are ongoing, but until they provide results, the aggressive approach is still preferred in high-risk patients.
Given the rapid evolution of these guidelines toward the invasive approach to management, several organizational issues have become important. There is a considerable body of evidence showing that volume and expertise are critical in delivering acute invasive care to patients with ACS of both types. These findings point to the possibility of developing “ACS centers,” similar to current trauma centers, so that well-prepared facilities can provide the best coordinated care.31 An additional facet of this issue is the question of on-site surgery. The recently completed Cardiovascular Patient Outcomes Research Team (C-PORT) trial32 found that direct PCI in sites without on-site surgery was superior to fibrinolysis with alteplase, although the trial was small and debate has arisen about whether these results are generalizable. Nevertheless, an increasing number of small hospitals are offering direct PCI for STEMI without on-site surgical backup. Arrangements should be in place at such institutions for rapid transfer of patients who need surgery, although the number of such cases continues to increase.
The role of surgery in this invasive approach remains subjective and poorly defined. In general, current guidelines recommend surgery for patients with three-vessel disease, left main disease, or concurrent structural defects (mitral regurgitation, septal defect). Despite the finding in the Bypass Angioplasty Revascularization Investigation (BARI) of the superiority of surgery over PCI in patients with diabetes mellitus,33 referral patterns continue to favor PCI in diabetic patients unless the anatomy is considered technically prohibitive. Continuously emerging innovations such as drug-eluting stents, robotic surgery, and combined procedures make exact determination of the indication for PCI versus bypass surgery fluid at present.