Mediastinal Tumor Markers
Philip G. Robinson
This chapter discusses the tumor markers, particularly the serum markers, of mediastinal tumors. Mediastinal tumors are a diverse group of tumors that share an anatomic location in the thorax. Using the anatomic divisions described in Chapter 162, the anterior mediastinum may be described as extending from the posterior sternum to the anterior aspect of the heart and anterior borders of the great vessels as they pass up to the thoracic inlet. The posterior mediastinum extends from the anterior border of the ventral longitudinal ligament of the thoracic vertebral column to the posterior chest wall and includes the paravertebral sulci. The middle mediastinum is the area between the anterior and posterior areas and includes the heart, great vessels, trachea, and esophagus. The tumors and tumor markers discussed in this chapter arise mostly in the anterior and posterior mediastinal compartments (Table 168-1). This chapter discusses the tumor markers of malignant germ cell tumors, thymomas, thymic carcinomas and carcinoids, neurogenic tumors, paragangliomas, and parathyroid tumors.
A tumor marker is a biological property of a neoplasm that indicates its presence. In most instances, tumor markers are not specific enough to establish a diagnosis. They are useful in (a) supporting a diagnosis, (b) determining response to therapy, (c) detecting a relapse, and (d) screening to detect a neoplasm at an early stage. They may be the expression of new gene products, altered amounts of normal gene products, alterations in chromosomal DNA, or one of many other structural or functional cellular properties. Tumor markers generally fall into one of three categories: tumor product, such as beta human chorionic gonadotropin (β-HCG); (b) cytogenetic alteration; and (c) molecular markers, such as an oncogene. Tumor markers are usually hormones, enzymes, intracellular proteins, or cell membrane antigens that can be detected or measured in serum, plasma, urine, or other body fluids. Until recently, the expression of new components by neoplasms has been the easiest way to study differences between normal and neoplastic tissues. The term tumor marker is often taken to mean the differences in antigenic expression between the neoplasm and normal tissues, but it should not be limited to this definition. For instance, in acute lymphocytic leukemia, the neoplastic cells contain surface proteins that appear in the normal development of lymphocytes but are inappropriate in the clinical context. The ideal tumor marker would be 100% specific and 100% sensitive. In addition,
it would indicate the degree of tumor burden, have prognostic value, be reproducible, and be easily measured in a cost-effective manner. To date, no tumor marker meets these criteria. The tumor marker that most closely approaches the ideal marker is the Bence–Jones protein, either the κ or λ light chains of immunoglobulins, which can be found in the urine of some patients with multiple myeloma.
it would indicate the degree of tumor burden, have prognostic value, be reproducible, and be easily measured in a cost-effective manner. To date, no tumor marker meets these criteria. The tumor marker that most closely approaches the ideal marker is the Bence–Jones protein, either the κ or λ light chains of immunoglobulins, which can be found in the urine of some patients with multiple myeloma.
Table 168-1 Mediastinal Tumors in the Anterior, Middle (Visceral), and Posterior Compartments That May Have Tumor Markers | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
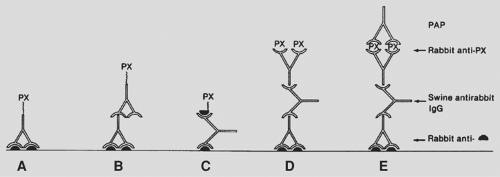
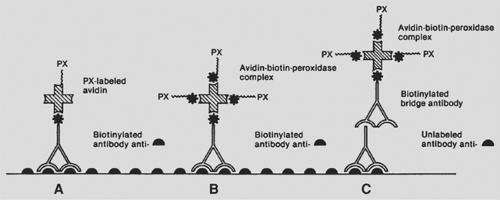
Tumor markers can be detected in serum or urine or in tissue by immunologic or cytogenetic methods. Serum or urine tumor markers include such markers as β-HCG, alpha-fetoprotein (AFP), catecholamines and their degradation products, chromogranin, and parathyroid hormone (PTH). Tissue tumor markers are tissue antigens, such as proteins or hormones, detected by immunologic techniques in fresh, frozen, or formalin-fixed, paraffin-embedded tissue sections. The technique consists of a primary reaction of an antibody to a tissue antigen and a subsequent reaction to visualize the antibody–antigen complex. The two most widely used methods are the peroxidase–antiperoxidase immune complex method and the avidin–biotin complex technique (Figs. 168-1 and 168-2). The mediastinal tumors with serum or urine tumor markers are shown in Table 168-2. The tumor markers for mediastinal lymphomas are discussed in Chapter 190.
Tumor Markers of Malignant Germ Cell Tumors
The incidence and different types of germ cell tumors and germ cell tumor markers are discussed below. Since germ cell tumors are more common in the gonads, this section draws on tumors of the gonads as well as those of the mediastinum. Malignant mediastinal germ cell tumors are uncommon, and their exact incidence is difficult to determine. Germ cell tumors also occur in the sacrococcygeal region, the orbit of the eye, the head (extracranial), the brain, and other sites along the midline. Economou and coworkers21 reviewed the files of Johns Hopkins Hospital from 1949 to 1980 and those of the University of California at Los Angeles from 1955 to 1980 for cases of malignant mediastinal germ cell tumors. They found 28 cases, of which 11 were pure seminomas and 17 nonseminomatous malignant germ cell tumors. In view of the population and referral bases of these two institutions, this small number of cases indicates that these tumors are extremely uncommon. Moran and Suster74 found 322 cases of primary mediastinal germ cell tumors in the files of the Armed Forces Institute of Pathology, Department of Pulmonary and Mediastinal Pathology, in Washington, DC, and Mount Sinai Medical Center of Greater Miami, Florida, from 1960 to 1994. Two of the patients were women and the remaining 320 were men. The two women had teratomatous lesions with malignant components. The analysis of the tumors yielded 37% seminomas, 16% yolk sac tumors (endodermal sinus tumor), 2% embryonal carcinomas, 2.5% choriocarcinomas, 3% combined nonteratomatous germ cell tumors, and 40% teratomatous tumors. Of the teratomatous tumors, 63% were mature teratomas (benign tumors), 33% were teratomas with additional malignant components, and 4% were immature teratomas.
Benign and malignant germ cell tumors are postulated to occur in the mediastinum either because some of the germ cells did not migrate properly to the genital ridges or possibly because a focus of pluripotential embryonic cells escaped the influence of their primary organizer during embryonic development. Another hypothesis is that they represent metastatic testicular lesions and the primary lesion has regressed. Benign and malignant germ cell tumors occur in both men and women, usually in the first three decades of life. The lesions are located in the
anterior compartment of the mediastinum. According to Malagon and Montiel,55 germ cell tumors account for 16% of mediastinal neoplasms in adults and between 19% and 25% in children. Mediastinal germ cell tumors are divided into prepubertal and postpubertal. In the prepubertal group only two types of germ cell tumors have been reported in the mediastinum: teratoma and yolk sac tumor. The anterior mediastinum is the third most common location after the sacrococcygeal region and the central nervous system. Postpubertal germ cell tumors have a striking predilection for men, with all types having been described. Hainsworth31 reminds us that these tumors have a particular predilection for men with Klinefelter’s syndrome (karyotype 47, XXY). Finally, patients with mediastinal germ cell tumors may develop hematologic malignancies.
anterior compartment of the mediastinum. According to Malagon and Montiel,55 germ cell tumors account for 16% of mediastinal neoplasms in adults and between 19% and 25% in children. Mediastinal germ cell tumors are divided into prepubertal and postpubertal. In the prepubertal group only two types of germ cell tumors have been reported in the mediastinum: teratoma and yolk sac tumor. The anterior mediastinum is the third most common location after the sacrococcygeal region and the central nervous system. Postpubertal germ cell tumors have a striking predilection for men, with all types having been described. Hainsworth31 reminds us that these tumors have a particular predilection for men with Klinefelter’s syndrome (karyotype 47, XXY). Finally, patients with mediastinal germ cell tumors may develop hematologic malignancies.
Table 168-2 Serum or Urine Markers of Mediastinal Tumors | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
In the past, mediastinal germ cell tumors were classified with the same system as the testicular germ cell tumors. Travis and colleagues110 in the World Health Organization (WHO) classification of lung tumors introduced a specific scheme for mediastinal germ cell tumors. This classification is based on the one described by Elbe and colleagues22 in the WHO classification of testicular tumors. Previously, Moran and Suster74 proposed a classification scheme specifically for mediastinal germ cell tumors. These two classification schemes are compared in Table 168-3. Occasionally the term teratocarcinoma is used to describe a teratoma with a focus of embryonal carcinoma, but it has also been applied to primary carcinomas arising in teratomas. The term should not be used in this manner; rather, the components of germ cell tumor should be named.
Klein45 reviewed the tumor markers associated with germ cell tumors; they are (a) AFP (yolk sac tumor or mixed tumors); (b) placental alkaline phosphatase (PLAP) (seminoma, yolk sac tumor, and embryonal carcinoma); and (c) β-HCG (choriocarcinoma). These are probably the three most important serum tumor markers in germ cell tumors. According to Billmire,8 the two most useful markers for germ cell tumors are alpha fetoprotein (AFP), secreted by yolk sac tumors, and β-HCG, secreted by choriocarcinomas. CD30 has been detected in serum but is also used as an immunohistochemical stain. The tumor markers that are useful in immunohistochemical staining of germ cell tumors are shown in Table 168-4. In the review of Malagon and Montiel,55 embryonal carcinomas do not stain immunohistochemically for AFP. Most likely the association of AFP and embryonal carcinoma occurred because the tumor also had a component of yolk sac tumor. Klein45 noted that β-HCG was also present in 80% of patients with embryonal carcinoma and in 10% to 25% of those with pure seminoma. Other serum or immunohistochemical tumor markers include lactic acid dehydrogenase (LDH), OCT4/3, NANOG, VASA, and c-kit (CD117). In this section the serum markers are discussed first. If such a marker is also an immunohistochemical marker, it is discussed there. At the end of the section, the immunohistochemical markers are discussed.
Serum and Immunohistochemical Markers
Alpha-Fetoprotein (AFP)
Abelev and coworkers1 first described AFP as a serum tumor marker in mouse hepatomas. AFP is a single-chain oncofetal glycoprotein with a molecular weight of approximately 70,000 Da. The liver, yolk sac, and gastrointestinal tract of the fetus synthesize it. By 1 year of age, AFP decreases to adult levels. It can be elevated in patients with liver disease, especially with liver cell regeneration and with neoplasms such as hepatocellular carcinoma, germ cell tumors, and various other carcinomas. AFP is not present in pure seminomas, choriocarcinomas, or embryonal carcinomas. When the histology shows one of these three tumors and the AFP is elevated, there is a component of yolk sac tumor.
Since the discovery of elevated AFP in yolk sac tumors, many investigators have studied its significance. Scardino and coworkers95 pointed out the value of AFP in the staging and prognosis of patients with these two types of malignant germ cell
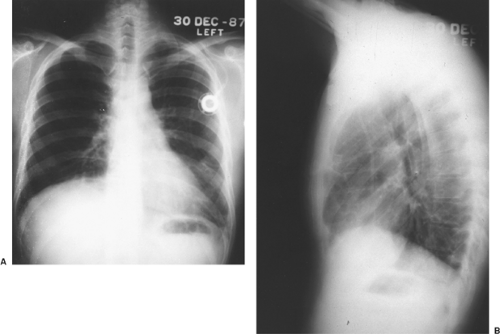
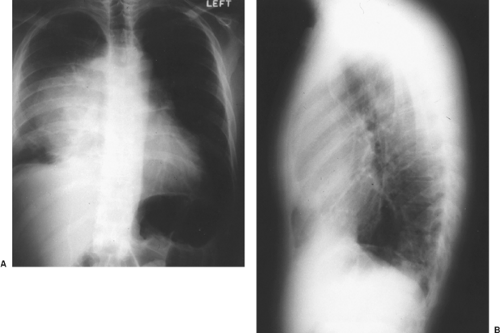
tumors of the testis. Kesler and associates44 found an elevated AFP level in nonseminomatous germ cell tumors >1,000 ng/mL after chemotherapy to be a negative predictor of survival. Wright and colleagues124 emphasized the association of normalization of serum tumor markers after chemotherapy for malignant germ cell tumor with a favorable prognosis (Fig. 168-3). Talerman107 and Perlin87 and their colleagues have pointed out the usefulness of AFP for following the activity of yolk sac tumors and embryonal carcinomas after they have been treated. AFP levels (and usually a tissue biopsy) are essential in evaluating young adult men with anterior mediastinal masses (Fig. 168-4). The biopsy might show only a teratoma, but an elevated AFP
level would indicate that yolk sac tumor is also present. Moran and associates71 immunostained 17 mediastinal yolk sac tumors, three embryonal carcinomas, two combined germ cell tumors containing embryonal carcinoma and yolk sac tumor, and one combined germ cell tumor containing yolk sac tumor and seminoma. The embryonal carcinomas and the yolk sac tumors both stained with low-molecular-weight cytokeratin, but the staining in the yolk sac tumors was focal. Immunostains for AFP were positive in 12 of 17 yolk sac tumors and negative in the three cases of embryonal carcinoma. Iczkowski and Bulter40 point out that the staining for AFP in yolk sac tumors is patchy. They also point out that embryonal carcinomas may have weak or focal staining for AFP.
tumors of the testis. Kesler and associates44 found an elevated AFP level in nonseminomatous germ cell tumors >1,000 ng/mL after chemotherapy to be a negative predictor of survival. Wright and colleagues124 emphasized the association of normalization of serum tumor markers after chemotherapy for malignant germ cell tumor with a favorable prognosis (Fig. 168-3). Talerman107 and Perlin87 and their colleagues have pointed out the usefulness of AFP for following the activity of yolk sac tumors and embryonal carcinomas after they have been treated. AFP levels (and usually a tissue biopsy) are essential in evaluating young adult men with anterior mediastinal masses (Fig. 168-4). The biopsy might show only a teratoma, but an elevated AFP
level would indicate that yolk sac tumor is also present. Moran and associates71 immunostained 17 mediastinal yolk sac tumors, three embryonal carcinomas, two combined germ cell tumors containing embryonal carcinoma and yolk sac tumor, and one combined germ cell tumor containing yolk sac tumor and seminoma. The embryonal carcinomas and the yolk sac tumors both stained with low-molecular-weight cytokeratin, but the staining in the yolk sac tumors was focal. Immunostains for AFP were positive in 12 of 17 yolk sac tumors and negative in the three cases of embryonal carcinoma. Iczkowski and Bulter40 point out that the staining for AFP in yolk sac tumors is patchy. They also point out that embryonal carcinomas may have weak or focal staining for AFP.
Table 168-3 Histologic Classifications of Mediastinal Germ Cell Tumors with a Comparison of the World Health Organization Classification to the Classification of Moran and Suster | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
Table 168-4 Immunohistochemical Staining Markers of Germ Cell Tumors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Beta Human Chorionic Gonadotropin (β-HCG)
Human chorionic gonadotropin is a glycoprotein with a molecular weight of approximately 38,000 Da. It is composed of two dissimilar polypeptide chains called alpha and beta chains. The alpha chain is identical to the alpha polypeptide chains of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and thyroid-stimulating hormone (TSH), whereas the beta chain is unique to human chorionic gonadotropin. β-HCG is produced by the syncytiotrophoblasts of the normal placenta. It is thought to have three functions: (a) maintenance of the corpus luteum during the first few weeks of pregnancy, (b) promotion of steroidogenesis of the fetal–placental unit, and (c) stimulation of the secretion of testosterone from the fetal testicle. The hormone is used as a serum marker for pregnancy and trophoblastic disease. However, it may be found in association with other tumors. Scardino and colleagues95 found that β-HCG was a valuable marker in detecting subclinical tumor recurrences. At a National Institutes of Health conference in 1979 moderated by Anderson,2 Waldmann pointed out that β-HCG was not only valuable in detecting tumor recurrences but also useful in measuring the effectiveness of the therapy the patient had received. As previously mentioned, Wright and colleagues123 emphasized the association of normalization of serum tumor markers after chemotherapy for a nonseminomatous germ cell tumor of the mediastinum with a favorable prognosis.
The literature on β-HCG levels in mediastinal choriocarcinomas is limited. Knapp and colleagues47 described seven patients with anterior mediastinal malignant germ cell tumors who had either pure choriocarcinomas or choriocarcinomas mixed with other malignant germ cell elements. The five patients who had urine pregnancy tests were positive for β-HCG. In two other patients, the authors followed the serum β-HCG; in these two patients the levels of β-HCG increased as the disease progressed and, with a satisfactory therapeutic response, the level was seen to decrease. In a patient with a suspected anterior mediastinal malignant germ cell tumor, a serum β-HCG level is essential in determining whether choriocarcinoma is a component of the germ cell tumor. Moran and Suster75 reported a series of eight pure mediastinal choriocarcinomas. The patients were all men ranging in age from 21 to 63 years. All died within 2 months of diagnosis, emphasizing the poor prognosis of this disease.
Immunohistochemical staining of choriocarcinomas for β-HCG is also a valuable diagnostic test. Niehans and coworkers80 reported that there was no major difference in the immunohistochemical staining of gonadal and extragonadal malignant germ cell tumors. Moran and associates71 performed immunostains on six mediastinal choriocarcinomas and the β-HCG stained only the multinucleated syncytiotrophoblast giant cells.
Immunohistochemical staining of choriocarcinomas for β-HCG is also a valuable diagnostic test. Niehans and coworkers80 reported that there was no major difference in the immunohistochemical staining of gonadal and extragonadal malignant germ cell tumors. Moran and associates71 performed immunostains on six mediastinal choriocarcinomas and the β-HCG stained only the multinucleated syncytiotrophoblast giant cells.
Placental Alkaline Phosphatase (PLAP)
Alkaline phosphatase comprises a group of four isoenzymes that come from the liver, bone, placenta, or intestine. The phosphatases are hydrolases of low specificity. These enzymes have a molecular weight of approximately 120,000 Da and function to catalyze the transfer of a phosphate group from a donor compound to an acceptor compound that contains a hydroxyl group. Placental syncytiotrophoblast cells produce the placental isoenzyme of alkaline phosphatase by the 12th week of pregnancy. This enzyme is also produced by seminomas. Lange and coworkers50 studied serum levels of PLAP in patients with seminomas and nonseminomatous malignant germ cell tumors. They concluded that PLAP was a clinically useful serum marker in patients with seminoma. The International Union Against Cancer Workshop on Immunodiagnosis42 noted that PLAP is also a useful serum and immunohistochemical marker in the management of seminomas. Tonik109 and Maslow60 and their coworkers, however, demonstrated that PLAP could also be elevated in patients who smoke cigarettes. Tucker and coworkers111 concurred with this finding and suggested that PLAP measurements might not be as useful in following patients who have seminomas and who also smoke.
Nielsen and colleagues81 evaluated PLAP as a serum tumor marker in both smokers and nonsmokers with seminomas. After careful evaluation, they concluded that PLAP was not a useful stand-alone marker for seminoma and contributed little to the follow-up of these patients. Subsequently, reports have appeared suggesting that PLAP may be of some value in patients with seminomas. Nielsen81 and Munro78 and their coworkers evaluated the combined use of PLAP, LDH, and β-HCG as follow-up tumor markers in patients with seminomas. They found that if either the β-HCG was >6 IU/L1 or the LDH was >400 IU/L1 and the PLAP was >60 IU/L1, this combination would detect approximately 50% of patients with disease. Koshida and colleagues48 found PLAP to be elevated at the time of initial diagnosis in 50% of patients with seminomas and nonseminomatous germ cell tumors. They concluded that PLAP might be useful in monitoring these patients. Weissbach and associates115 used β-HCG, LDH, and PLAP to monitor seminomas before and after orchiectomy and in follow-up. They concluded that all three markers should be determined in patients with seminomas. PLAP was most likely to be elevated and it was the most sensitive in detecting metastatic disease. In summary, the most recent reports suggest that PLAP in conjunction with other markers may be of value in monitoring seminomas.
Burke and Mostofi11 detected PLAP immunohistochemically in the tissue of other malignant germ cell tumors. They found
that 96% of seminomas stained positively for PLAP but also that other benign and malignant germ cell tumors stained with PLAP in varying percentages. The percentage of the other germ cell tumors that stained with PLAP were as follows: embryonal carcinoma, 96%; choriocarcinoma, 45%; syncytiotrophoblast, 43%; yolk sac tumor, 25%; mature teratoma, 5%; and immature teratoma, 4%. The study of Niehans and coworkers81 noted that extragonadal malignant germ cell tumors did not immuno- stain significantly differently from those of the gonads. Moran and associates70 immunostained 50 mediastinal seminomas for PLAP and found 80% of them to be strongly positive. The low-molecular-weight cytokeratin CAM 5.2 was positive in 75% of the cases and had focal dot-like positivity. The broad-spectrum cytokeratin was positive in 70% of the cases. In approximately 5% of the cases, β-HCG was present in individual seminoma cells. No immunostaining was observed for AFP, epithelial membrane antigen, or carcinoembryonic antigen.
that 96% of seminomas stained positively for PLAP but also that other benign and malignant germ cell tumors stained with PLAP in varying percentages. The percentage of the other germ cell tumors that stained with PLAP were as follows: embryonal carcinoma, 96%; choriocarcinoma, 45%; syncytiotrophoblast, 43%; yolk sac tumor, 25%; mature teratoma, 5%; and immature teratoma, 4%. The study of Niehans and coworkers81 noted that extragonadal malignant germ cell tumors did not immuno- stain significantly differently from those of the gonads. Moran and associates70 immunostained 50 mediastinal seminomas for PLAP and found 80% of them to be strongly positive. The low-molecular-weight cytokeratin CAM 5.2 was positive in 75% of the cases and had focal dot-like positivity. The broad-spectrum cytokeratin was positive in 70% of the cases. In approximately 5% of the cases, β-HCG was present in individual seminoma cells. No immunostaining was observed for AFP, epithelial membrane antigen, or carcinoembryonic antigen.
Lactic Acid Dehydrogenase (LDH)
Lactic acid dehydrogenase is an enzyme that helps with the oxidation of lactic acid to pyruvic acid. It has a molecular weight of 138,000 Da and is composed of four isoenzymes. According to Klein,45 80% of patients with advanced malignant germ cell tumors have elevated levels of LDH. The elevation is nonspecific and is seen with all types of malignant germ cell tumors. Carver and Sheinfeld13 believe that elevated serum LDH is useful in clinical decision making and that it correlates with tumor burden, growth rate, and cellular proliferation. They state that LDH is elevated in 80% of patients with metastatic seminoma and in about 60% of patients with advanced nonseminomatous germ cell tumors. In contrast, Venkitaraman and colleagues112 thought that serum LDH had limited specificity, sensitivity, and positive predictive value for detecting relapse of testicular germ cell tumors. von Eyben and colleagues113 found that patients with metastatic testicular germ cell tumor who had an elevated level of isoenzyme 1 had a poorer prognosis than other patients.
CD30
CD30 was initially described as a diagnostic marker for Hodgkin’s disease and anaplastic lymphoma. Latza and associates51 detected soluble CD30 antigen in the sera of eight of eight patients with embryonal carcinoma. They did not detect it in the sera of 8 of 10 patients with other testicular germ cell tumors. They concluded that a serum level of CD30 could be a useful test for monitoring patients with embryonal carcinoma. To date, this marker has not gained widespread use.
In 1994, Ferreiro,25 using immunohistochemical techniques, detected the antigen in 88% of embryonal carcinomas and 83% of embryonal carcinoma elements in mixed malignant germ cell tumors. None of the seminomatous, yolk sac tumor, or teratomatous components expressed CD30. Suster and colleagues,106 using immunohistochemistry, found CD30 in many embryonal carcinomas but also in some yolk sac tumors and seminomas. Both reports concluded that CD30 would be a helpful immunohistochemical marker for embryonal carcinoma. Iczkowski and colleagues41 pointed out that CD30 does not have as high specificity as OCT3/4 when used to stain embryonal carcinomas.
Immunohistochemical Markers
D2-40
Lau and colleagues52 used D2-40 as an immunohistochemical tumor marker in a study to differentiate seminoma from embryonal carcinoma. D2-40 is a monoclonal antibody that reacts with a 40,000-Da O-glycosylated sialoglycoprotein that is an oncofetal antigen know as M2A. The antigen is present in the fetal germ cells of the testis as well as in the lymphatic endothelial cells and mesothelial cells. M2A has been shown to be restricted to seminoma and intratubular germ cell neoplasia. Lau and colleagues52 thought that it might be useful in distinguishing seminoma from embryonal carcinoma. They found that all of their seminomas stained with this antigen, but that 4 of 14 (29%) embryonal carcinomas also stained for it. Iczkowski and associates41 found that 23 out of 24 (95.8%) seminomas stained with D2-40 and 6 out of 17 (35%) embryonal carcinomas stained. The exact role of D2-40 in differentiating seminoma from embryonal carcinoma is unclear.
C-kit (CD117)
Iczkowski and associates41 used c-kit as an immunohistochemical tumor marker in a study to differentiate seminoma from embryonal carcinoma. C-kit is a transmembrane glycoprotein that promotes dimerization and autophosphorylation of KIT receptors. KIT signaling is critical for the normal development and survival of germ cells. Membranous expression of c-kit is fairly constant in seminoma and intratubular germ cell neoplasia. Iczkowski and associates41 found that 17 out of 22 (77%) embryonal carcinomas stained with c-kit and that 40 out of 41 (98%) seminomas stained with c-kit. The former showed a cytoplasmic staining pattern, whereas the latter showed a membranous pattern. They concluded that c-kit is useful for establishing a diagnosis of extragonadal seminoma but thought that it should be used in conjunction with another marker, such as D2-40. They also concluded that in conjunction with CD30, c-kit might be useful in differentiating seminoma from embryonal carcinoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree