INTRODUCTION
An adequate pathologic assessment of lymph nodes draining the site of the primary tumor is an important oncologic principle in nearly all solid organ malignancies. Complete histologic evaluation of lymph nodes provides significant prognostic information for overall survival and disease-free survival. Additionally, the overall number of resected nodes has become a quality measure of the adequacy of surgical resection. Pathologic lymph node status (pN) is a crucial component of the tumor, node, and metastasis (TNM) staging system and influences clinical and therapeutic decisions in patients with nonsmall cell lung cancer (NSCLC). Although the survival benefit of lymphadenectomy has not been proven in randomized trials, it is possible that a select group of patients with truly loco regional disease might benefit from a meticulous lymph node dissection beyond the merits such a dissection would have for stage classification. A surgeon’s role in this process is irreplaceable, as the performance of lymphadenectomy requires a thorough anatomic knowledge of lymph node basins draining the primary tumor site as well as a technical skill and judgment for safe accomplishment of this task.
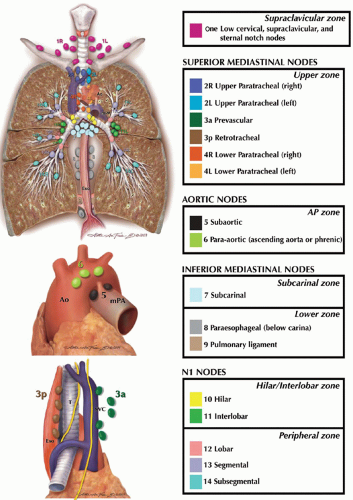
ANATOMY AND CLASSIFICATION OF INTRATHORACIC LYMPH NODES
The prognostic importance of the metastases to regional lymph nodes in patients with lung cancer has been recognized for over 50 years. The first comprehensive classification of the thoracic lymph node stations has been developed by Naruke et al.; this Japanese classification was used worldwide for almost four decades. In the 1990s, the American Thoracic Society (ATS) attempted to refine the anatomical descriptions of thoracic lymph nodes and in 1997, Mountain and Dresler published a modification of the ATS lymph node map, which was later implemented into the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) Lung Cancer Staging System. While this revised form of lymph node stations has been widely adopted in the United States, it was only sporadically used by the European surgeons, and the Japanese continued to favor the original Naruke’s classification. An effort to consolidate worldwide lung cancer staging data began in 1998 by the International Association for the Study of the Lung Cancer (IASLC) with the establishment of the Lung Cancer Staging Project. In this project, data from over 100,000 patients were collected. The discrepancies between Naruke and Mountain-Dresler thoracic lymph node maps became significant during data analysis as some patients with N2 (or stage IIIA) disease according to Mountain-Dresler classification were staged as N1 (or stage II) disease in Naruke classification. Consequently, IASLC published the third detailed anatomic classification of intrathoracic lymph node stations, reconciling the differences between former Japanese and American lymph node maps (
Table 30.1). Fourteen discrete lymph node stations have been recognized, which are grouped into the following seven zones: supraclavicular (level 1 nodes), superior mediastinal upper zone (level 2, 3, and 4 nodes), aortic nodes/aortopulmonary zone (level 5 and 6 nodes), inferior mediastinal subcarinal zone (level 7 nodes) and inferior mediastinal lower zone (level 8 and 9 nodes), N1 nodes hilar/interlobar zone (level 10 and 11 nodes), and peripheral zone (level 12, 13, and 14 nodes;
Fig. 30.1).
INTRATHORACIC LYMPHATIC FLOW AND METASTATIC SPREAD
The anatomical description of the mediastinal lymphatic flow may be credited to Riquet et al. who, in a meticulous postmortem dissection utilizing dye injection of the lymphatics, identified intrathoracic lymphatic drainage pathways. The in vivo investigation of the thoracic lymphatic flow was later studied by Hata et al., who generated dynamic lymphoscintigrams by injecting 99mTc-labeled colloid into the segmental bronchial submucosa. He identified the following lobar and segmental lymphatic drainage patterns; Right lung: the apical and posterior segments of the right upper lobe drained via the hilar nodes (level 10), tracheobronchial nodes (level 4), and upper paratracheal lymph nodes (level 2) into the ipsilateral scalene nodes. Drainage from the anterior segment of the right upper lobe varied with approximately 50% of flow via subcarinal (level 7) lymph nodes into the right scalene nodes; occasionally, the lymph flow crossed the midline following the course of the innominate vein into the left scalene lymph nodes. The other half of time the flow from the anterior segment resembled the flow the other segments of the upper lobe. Lymphatic drainage from the middle lobe and the superior segment of the lower lobe resemble the upper lobe drainage ultimately ending in the ipsilateral scalene nodes either via lower paratracheal or subcarinal nodes. Again, in a minority of cases the flow from the middle and lower lobes was noted to cross to the left scalene nodes via subcarinal and left lower paratracheal nodes. Left lung: Lymphatic flow from the left lung was variable; however, certain patterns were identified. The apical posterior segment of the left upper lobe drained primarily via the subcarinal lymph nodes along the left vagus nerve to the left scalene nodes or along the recurrent laryngeal to the paratracheal lymph nodes. The anterior and lingular segments drained along the left nerve through the para-aortic nodes (level 5/6) to the ipsilateral scalene lymph nodes. Lymph from the basilar segments flowed via the subcarinal nodes to the pretracheal and contralateral paratracheal lymphatics to the right scalene nodes. Drainage from the superior segment was least constant and occurred via multiple pathways. The study demonstrated mostly consistent ipsilateral lymph drainage along mediastinal lymph node stations with an occasional contralateral drainage.
A number of authors have studied the pattern of intrathoracic metastatic spread of lung cancer. Borrie initially demonstrated cancer occurrence along the bronchus intermedius on the right side and along the main fissure on the left; these lymphatic sumps are now referred to as the Sumps of Borrie. In a study of 359 patients who underwent mediastinoscopy, scalene node biopsy and thoracotomy, Nohl-Oser identified the pattern of regional metastatic spread. Right upper lobe tumors had a propensity to spread to ipsilateral mediastinal nodes (75%); contralateral mediastinal or scalene node involvement was rare in both right upper and lower lobe tumors (<5% to 7%). Left upper lobe tumors, however, had higher incidence of contralateral involvement in mediastinal and scalene nodes (10% to 13%) as did left lower lobe tumors (14% scalene and 25% mediastinal).
Intrathoracic lymphatic involvement of biopsy-proven N2 disease was characterized by Asamura et al. in a study of 166 patients. Right upper and lower lobe tumors had much higher propensity to spread into the pretracheal and ipsilateral paratracheal nodes, whereas right middle lobe tumors had the highest association with subcarinal lymphadenopathy (88%). On the left side, metastatic disease was most consistently found in the aortopulmonary window and para-aortic nodes (levels 5 and 6). Involvement of subcarinal (level 7) nodes occurred in the fifth of patients, most frequently when a tumor involved the lingular segment. Metastatic disease from lower lobe tumors was found with almost equal frequency in the subcarinal and aortopulmonary nodes. Although these studies demonstrate
frequent orderly ipsilateral cancer spread from the intraparenchymal to hilar to paratracheal lymph node chains, skip metastases may occur in up to one-third of cases. The occasional drainage into the contralateral lymph nodes argues for a vigilant preoperative lymph node evaluation in patients with newly diagnosed lung cancer.