Using optical coherence tomography (OCT), the mechanisms of postintervention and 9-month luminal enlargement in drug-eluting stent in-stent restenosis (ISR) lesions treated with a drug-eluting balloon (DEB) were evaluated. A total of 42 patients with DEB-treated drug-eluting stent ISR lesions underwent serial OCT examination before intervention, after intervention, and at 9-month follow-up. Preintervention OCT-derived neointima was classified as either a homogeneous or nonhomogeneous pattern. Ten ISR lesions with homogeneous neointima were identified and compared with 32 ISR lesions with nonhomogeneous neointima. When comparing pre- and postintervention evaluations, changes in luminal cross-sectional area (CSA) were 3.4 mm 2 in ISR lesions with homogeneous neointima and 3.7 mm 2 in those with nonhomogeneous neointima, respectively (p = 0.529); changes in stent CSA were 2.5 mm 2 and 1.4 mm 2 , respectively, p = 0.004; and changes in neointimal CSA were −0.9 mm 2 and −2.3 mm 2 , respectively, p = 0.001. At 9-month follow-up, changes in luminal CSA were −2.0 mm 2 and −0.9 mm 2 in ISR lesions with homogeneous and nonhomogeneous neointima, respectively (p = 0.021); in stent CSA changed by −0.2 mm 2 in both groups (p = 0.851) and changes in neointimal CSA was 1.8 mm 2 and 0.7 mm 2 , respectively (p = 0.003). At the 9-month follow-up, >50% neointimal CSA stenosis was observed in 60% and 19% of the ISR lesions with homogeneous and nonhomogeneous neointima, respectively (p = 0.020). In conclusion, the mechanism of postintervention luminal enlargement by DEB varied with the preintervention OCT-based neointimal characteristics. ISR lesions with homogeneous neointima determined by OCT were associated with greater subsequent regrowth of neointima after DEB treatment.
For the treatment of in-stent restenosis (ISR), drug-eluting balloon (DEB) angioplasty has been consistently demonstrated to be more effective than plain balloon angioplasty. Furthermore, angiographic and clinical outcomes of DEB angioplasty are comparable with those of paclitaxel-eluting stent implantation in randomized clinical trials. However, a recent large-scale prospective registry study demonstrated that the occurrence of repeated revascularization after DEB angioplasty was more frequent in drug-eluting stent (DES) ISR lesions compared with bare-metal stent (BMS) ISR lesions. Although these findings suggested that the vascular responses of DES ISR may differ from those observed in BMS ISR, the underlying mechanism of this DEB-dependent effect on DES ISR lesions is not known. Optical coherence tomography (OCT) is able to visualize the restenotic neointimal tissue inside coronary stents more clearly than intravascular ultrasound. Therefore, using serial OCT examination, the present study sought to evaluate the mechanism of postintervention and 9-month luminal enlargement in DES ISR lesions treated with a DEB by examining how the neointimal pattern of the ISR lesion impacts this outcome.
Methods
A total of 65 patients who underwent DEB angioplasty for ISR lesions, who also had pre- and postintervention OCT examinations, were retrospectively identified from the OCT registry of our institute. The DEB angioplasty for ISR lesions was performed for documented myocardial ischemia with >50% of diameter stenosis by visual estimation. A 9-month follow-up OCT was performed in 62 patients. Twenty-three patients were excluded for the following reasons: 9-month follow-up OCT was not done in 3 patients, BMS ISR lesions were observed in 8 patients, poor quality of OCT images or unmatched OCT images was found in 6 patients, additional stent implantation was required in 5 patients, and 1 patient presented with stent thrombosis. Therefore, 42 patients were finally enrolled in this study. General inclusion and exclusion criteria to perform OCT in our institute have been previously described. This study was approved by the institutional review board of our institution, and written consent was obtained from all enrolled patients.
All patients received at least 75 mg of aspirin and a loading dose of 300 mg of clopidogrel at least 12 hours before DEB angioplasty. During the intervention, unfractionated heparin was administered to maintain an activated clotting time >250 seconds. The DEB angioplasty procedures were performed according to current standard techniques. A paclitaxel-coated balloon (Sequent Please; B. Braun, Melsungen, Germany) was inflated for 60 seconds after ISR lesions were predilated with a plain balloon. An adequate size of the paclitaxel-coated balloon was determined with consideration to the length of target ISR lesion and the diameter of the previously used DESs. After procedure, dual antiplatelet therapy of 100 mg/day aspirin indefinitely and 75 mg/day clopidogrel for at least 1 month was prescribed.
Quantitative coronary angiography analysis was performed before and after stent implantation and at follow-up using an off-line quantitative coronary angiographic system (CASS system; Pie Medical Imaging BV, Maastricht, the Netherlands) in an independent core laboratory (Cardiovascular Research Center, Seoul, Korea). The angiographic pattern of ISR followed Mehran’s classification. Binary restenosis was defined as stenosis >50% of the luminal diameter. Late loss was defined as the difference between the postintervention and 9-month follow-up minimal luminal diameters.
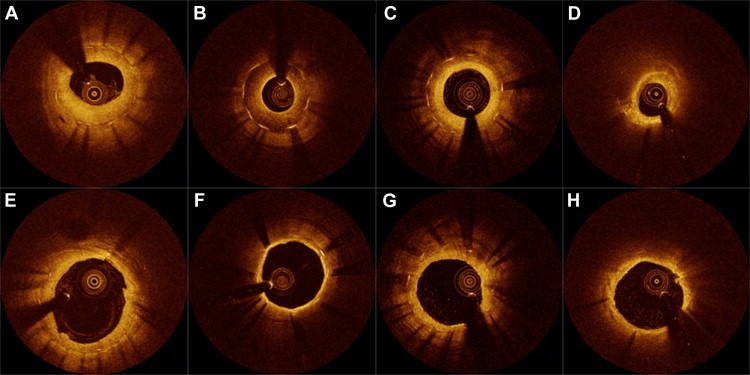
OCT imaging of DES ISR lesions was performed before intervention, after intervention, and at the 9-month follow-up using a frequency-domain C7-XR OCT system (St. Jude Medical, St. Paul, Minnesota). Detailed OCT procedures and analysis were described in a previous report. All OCT images were analyzed with off-line software (QIvus; Medis Medical Imaging System, Leiden, the Netherlands) at the same core laboratory as angiography. After all cross-sectional images on preintervention OCT were quantitatively measured at 1-mm intervals, consecutive cross sections with a percentage of cross-sectional area (CSA) stenosis of neointima >50% were selectively identified. These cross sections were serially matched with those after intervention and at follow-up using physical landmarks such as stent edges or bifurcations. The stented segments with the maximal percentage of neointimal CSA stenosis were then qualitatively assessed to classify the neointimal tissue into the following types: (1) homogeneous neointima, a uniform signal-rich band without focal variation or attenuation and (2) nonhomogeneous neointima with (a) simple nonhomogeneous neointima (focally changing optical properties and various backscattering patterns), (b) layered neointima (layers with different optical properties, namely an adluminal high-scattering layer and an abluminal low-scattering layer), or (c) neoatherosclerotic neointima (lipid-laden neointima, thin-cap fibroatheroma-like neointima, or neointima with calcification) ( Figure 1 ). Neointimal dissection within the stent was defined as the disruption of neointimal continuity after DEB angioplasty in neointimal tissue that was intact before the intervention took place. In the present study, 10 patients with homogeneous neointima were compared with the other 32 patients with nonhomogeneous neointima.
Statistical analysis was performed using PASW (version 18.0.0; SPSS Inc., Chicago, Illinois). Data are expressed as number (percentage), mean ± SD, or median (interquartile range). Comparisons of categorical data were made using chi-square statistics, Fisher’s exact test, or McNemar test. Continuous variables were compared using Student t test or the Mann-Whitney U test. Paired continuous variables were compared using repeated-measures analysis of variance with Bonferroni correction for post hoc tests. Changes between ISR lesions with homogeneous and nonhomogeneous neointima were compared using multivariate regression analysis to adjust baseline CSA. A p value of <0.05 was considered statistically significant.
Results
Baseline characteristics of the 42 patients included in this study are listed in Table 1 . Serial angiographic and OCT findings are listed in Table 2 . The average time between the DEB procedure and follow-up angiography for the entire population was 9.5 ± 2.4 months; this time interval was not statistically different between groups (9.0 ± 1.4 months, homogeneous group and 9.7 ± 2.7 months, nonhomogeneous group, p = 0.723). Binary angiographic restenosis was noted in 8 patients (19%) at follow-up with 0.41 ± 0.64 mm of angiographic late loss. Six of 42 patients (14%) underwent repeated revascularization. Between before and after interventions, stent CSA increased from 6.5 ± 1.3 to 8.1 ± 1.6 mm 2 (p <0.001), whereas neointimal CSA decreased from 4.4 ± 1.3 to 2.4 ± 0.9 mm 2 (p <0.001). Therefore, luminal CSA increased from 2.1 ± 0.7 to 5.7 ± 1.3 mm 2 (p <0.001). The luminal enlargement after DEB angioplasty (3.6 ± 1.2 mm 2 ) resulted from an increase in stent CSA of 1.6 ± 1.0 mm 2 (46 ± 24%) and a decrease in neointimal CSA of 2.0 ± 1.2 mm 2 (54 ± 24%). Between after intervention and follow-up, luminal CSA decreased from 5.7 ± 1.3 to 4.6 ± 1.7 mm 2 , p <0.001, in conjunction with a slight decrease in stent CSA (0.2 ± 0.2 mm 2 ) and a prominent increase in neointimal CSA (0.9 ± 1.3 mm 2 ). Compared with preintervention OCT, homogeneous neointima was more frequently observed on follow-up OCT (24% vs 64%, p = 0.001). Neointimal dissection detected on the postintervention OCT completely disappeared on follow-up OCT.
| Variable | Total (n = 42) | Homogeneous Neointima | p Value | |
|---|---|---|---|---|
| Yes (n = 10) | No (n = 32) | |||
| Age (yrs) | 65.3 ± 9.3 | 63.3 ± 9.4 | 66.0 ± 9.3 | 0.535 |
| Men | 35 (83) | 10 (100) | 25 (78) | 0.168 |
| Hypertension | 34 (81) | 7 (70) | 27 (84) | 0.369 |
| Diabetes mellitus | 21 (50) | 3 (30) | 18 (56) | 0.147 |
| Current smoker | 6 (14) | 1 (10) | 5 (16) | 1.000 |
| Renal dysfunction | 1 (2) | 0 (0) | 1 (3) | 1.000 |
| Clinical presentation | 0.236 | |||
| Stable angina pectoris | 38 (90) | 8 (80) | 30 (94) | |
| Acute coronary syndrome | 4 (10) | 2 (20) | 2 (6) | |
| Stent type | 0.604 | |||
| Sirolimus-eluting stent | 10 (24) | 2 (20) | 8 (25) | |
| Paclitaxel-eluting stent | 5 (12) | 0 (0) | 5 (16) | |
| Zotarolimus-eluting stent | 13 (31) | 3 (30) | 10 (31) | |
| Everolimus-eluting stent | 9 (21) | 3 (30) | 6 (19) | |
| Biolimus A9–eluting stent | 5 (12) | 2 (20) | 3 (9) | |
| Stent diameter (mm) | 3.1 ± 0.3 | 3.0 ± 0.2 | 3.1 ± 0.3 | 0.350 |
| Stent length (mm) | 34.5 ± 17.9 | 36.9 ± 21.1 | 33.9 ± 17.4 | 0.588 |
| Overlapping stent | 13 (31) | 3 (30) | 10 (31) | 1.000 |
| Stent age (mo) | 21.0 (11.9–41.2) | 23.0 (12.8–42.2) | 15.2 (11.7–41.5) | 0.516 |
| Narrowed coronary artery | 0.362 | |||
| Left anterior descending | 33 (79) | 7 (70) | 26 (81) | |
| Right | 9 (21) | 3 (30) | 6 (19) | |
| Pattern of ISR | 0.416 | |||
| Focal | 33 (79) | 9 (90) | 24 (75) | |
| Diffuse | 9 (21) | 1 (10) | 8 (25) | |
| Predilation balloon diameter (mm) | 3.0 ± 0.3 | 3.1 ± 0.3 | 3.0 ± 0.3 | 0.658 |
| DEB diameter (mm) | 3.1 ± 0.3 | 3.2 ± 0.3 | 3.1 ± 0.3 | 0.460 |
| DEB length (mm) | 22.5 ± 5.6 | 22.1 ± 5.5 | 22.6 ± 5.7 | 0.856 |
| DEB/stent diameter ratio | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.507 |
| Inflation pressure of DEB (atm) | 10.2 ± 2.9 | 10.0 ± 2.4 | 10.3 ± 3.0 | 0.550 |
| Maximal final pressure (atm) | 15.7 ± 3.8 | 15.6 ± 3.6 | 15.7 ± 3.9 | 0.908 |
| Variable | Before Intervention | After Intervention | 9-Month Follow-Up | p Value |
|---|---|---|---|---|
| Angiography | ||||
| Reference vessel diameter (mm) | 2.81 ± 0.47 | 2.92 ± 0.51 | 2.89 ± 0.36 | 0.220 |
| Minimal luminal diameter (mm) | 0.90 ± 0.45 | 2.31 ± 0.54 | 1.92 ± 0.60 | <0.001 |
| Diameter stenosis (%) | 69.0 ± 15.5 | 20.4 ± 10.3 | 33.4 ± 20.0 | <0.001 |
| OCT | ||||
| Neointimal thickness (μm) | 608.8 ± 157.4 | 255.8 ± 91.8 | 385.5 ± 213.0 | <0.001 |
| Luminal CSA (mm 2 ) | 2.1 ± 0.7 | 5.7 ± 1.3 | 4.6 ± 1.7 | <0.001 |
| Stent CSA (mm 2 ) | 6.5 ± 1.3 | 8.1 ± 1.6 | 7.9 ± 1.5 | <0.001 |
| Neointimal CSA (mm 2 ) | 4.4 ± 1.3 | 2.4 ± 0.9 | 3.3 ± 1.4 | <0.001 |
| Percentage of neointimal CSA stenosis (%) | 66.9 ± 10.0 | 29.9 ± 8.8 | 41.9 ± 18.2 | <0.001 |
| Pattern of neointimal tissue | 0.001 | |||
| Homogeneous | 10 (24) | — | 27 (64) | |
| Nonhomogeneous | 32 (76) | — | 15 (36) | |
| Neointimal dissection | — | 40 (95) | 0 (0) | — |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


