Mechanical Complications of Myocardial Infarction
Gregory Janis
Sandeep Joshi
Eyal Herzog
Acute myocardial infarction (AMI) affects 1.7 million people in the United States. Mechanical complications of AMI result in some of the deadliest outcomes. It is difficult to assess the true incidence of these complications as both clinical and autopsy series differ considerably. Nevertheless, they are thought to be responsible for about 15% of all AMI deaths.1 It is important to realize that these catastrophic events can occur within minutes to hours after the inciting event or even days to weeks later. Mechanical complications of AMI can be subdivided into two major categories: acute phase and chronic phase. These phases are further broken down into the seven major topics of this chapter (Table 10.1).
LEFT VENTRICULAR FREE WALL RUPTURE
Left ventricular free wall rupture (LVFWR) is almost a fatal complication of myocardial infarction (MI). Despite great progress in the reduction of both mortality and morbidity from AMI, death related to LVFWR has been high.
In patients who die after an AMI many large studies have found a 14% to 26% incidence of cardiac rupture.2 According to the National Registry of Myocardial Infarction, which reviewed data from 350,755 patients, the incidence of cardiac rupture was <1%.3 Approximately 50% of the time, myocardial rupture occurs within the first 5 days after MI and within 2 weeks in >90% of cases.4,5 Regardless, acute or subacute myocardial rupture is a serious and predominantly fatal complication of acute MI.6
Studies have also suggested a higher mortality from free wall rupture with thrombolytics, as cardiac rupture was responsible for 7.3% of all deaths and 12.1% with thrombolytic agents. Thrombolytic therapy does not necessarily increase the risk of rupture but it may accelerate occurrence, often within the first 24 hours of drug administration.7 In contrast, the incidence of cardiac rupture may be lower in patients treated with percutaneous coronary intervention. In an observational study of 1,375 patients who received a thrombolytic agent or underwent primary angioplasty, the incidence of rupture was 3.3% and 1.8%, respectively.8 It is well known that myocardial rupture after MI is less common in patients with successful reperfusion.9
TABLE 10.1 Acute Phase vs. Chronic Phase Complications of Acute Myocardial Infarction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
One study found that myocardial rupture was 9.2 times more likely to occur in patients who had no prior history of angina or MI, ST-segment elevation or Q-wave development on the initial ECG, and peak MB-creatine kinase was >150 IU per L.4 The absence of collateral blood flow, demonstrated by the lack of prior ischemic symptoms, and the greater the infarct territory correlate with a higher risk of myocardial rupture. Other risk factors for rupture include anterior location of the infarction, age >70, and female sex.8,10
PATHOPHYSIOLOGY
Myocardial rupture more frequently involves the left ventricle than the right ventricle and seldom involves the atria.3 The infarct commonly affects the anterior and lateral walls of the left ventricle near the junction of the infarct and normal myocardium. Ventricular free wall rupture is defined as an acute, traumatic perforation of the ventricles, which may include pericardial rupture.6 With unsuccessful or no reperfusion, coagulation necrosis develops within the first 3 to 5 days.5 As the necrosis progresses, neutrophils infiltrate the myocardial space and release lytic enzymes that disintegrate the necrotic myocardium leading to perforation. The rupture thus is a result of transmural infarction, and the actual perforation can range in size from millimeters to centimeters depending on infarct size.5 Early phase rupture is defined as within 72 hours post-MI and late rupture is >4 days.
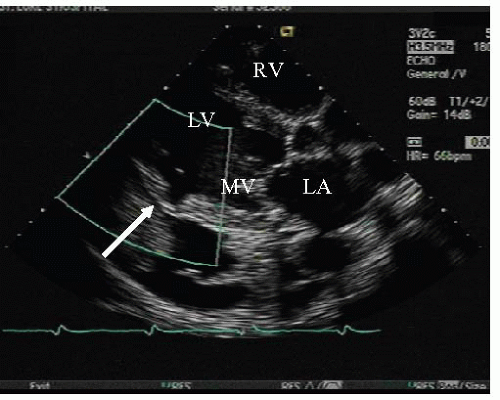
The hallmark of rapid deterioration is primarily because of the extravasation of blood into the pericardial space with resultant instantaneous, acute pericardial tamponade. Because these events occur so quickly, patients usually deteriorate before any therapeutic intervention. Less commonly, subacute rupture can provide a longer therapeutic window as the acute rupture may be temporarily contained by pericardial adhesions or by thrombosis at the rupture site. It is in these patients that immediate, lifesaving, cardiovascular surgery is possible (Figure 10.1).11
CLINICAL PRESENTATION
The clinical course of myocardial rupture is variable and challenging to diagnose, as patients may rapidly decompensate. Their clinical course parallels that of any patient suffering from pericardial tamponade. Thus, rupture can present as sudden death in an undetected or silent MI or as incomplete/subacute rupture in those with known MI.12
Complete rupture of the left ventricular free wall usually leads to hemopericardium and death from cardiac tamponade. Patients may complain of transient chest pain or dyspnea. Acute tamponade can cause tachypnea, tachycardia, muffled heart sounds, elevated jugular venous pressure, and pulsus paradoxus. The presence of rupture is first suggested by the development of sudden profound right heart failure and shock, often progressing rapidly to pulseless electrical activity and death. Emergent transthoracic echocardiography (TTE) will confirm the diagnosis and emergent pericardiocentesis can transiently relieve the tamponade.12
Incomplete/subacute rupture of the left ventricular free wall can occur when organized thrombus and the pericardium seal the ventricular perforation. Patients may present with persistent or recurrent chest pain (particularly pericardial pain), nausea, restlessness, agitation, abrupt hypotension, and/or electrocardiographic features of localized or regional pericarditis.4,5 The diagnosis of incomplete/subacute rupture is again confirmed by TTE.12
TREATMENT
As mentioned, the mortality for acute and subacute free wall rupture is high owing to rapid clinical deterioration. Survival depends primarily on the prompt recognition of myocardial rupture and provision of immediate therapy. Patients displaying suggestive symptoms, signs, and ECG changes require emergent bedside echocardiogram and echocardiographically guided pericardiocentesis if fluid is visualized. Immediate surgical intervention is indicated if the pericardiocentesis identifies the fluid as blood. Medical therapy aimed at hemodynamic stabilization should also be instituted. In addition to pericardiocentesis this includes fluids, inotropic support, vasopressors, and even intra-aortic balloon pump (IABP) counterpulsation and percutaneous cardiopulmonary bypass when available and indicated.4
With rapid recognition and initiation of both medical and surgical therapies, the potential for survival, particularly with subacute rupture, can improve dramatically. In one study 25 of 33 patients (76%) with subacute ventricular rupture survived the surgical procedure and 16 (48%) were long-term survivors.13
VENTRICULAR SEPTAL RUPTURE
Another deadly complication of AMI involves rupture of the interventricular septum. The need for rapid diagnosis, aggressive medical therapy, and prompt surgical intervention is essential to increase the possibility of survival. As medical therapy has advanced, the incidence and timing of ventricular septal rupture has been up for debate.
In the era before reperfusion therapy, rupture of the interventricular septum is thought to have occurred in 1% to 2% of patients with AMI and accounts for approximately 5% of deaths in this setting.14 It typically occurs in the first week after infarction, with a mean-time from symptom onset of 3 to 5 days.15 The classic risk factors for septal rupture in the pre-reperfusion era include hypertension, advanced age, female sex, and the absence of a history of MI or angina.16 Prognosis for ventricular septal rupture in the pre-reperfusion era was very poor, with an in-hospital mortality of about 45% in those treated surgically and about 90% in those treated medically.15 With the advent of thrombolysis and percutaneous intervention, outcomes have changed.
In the reperfusion era, studies show a much lower incidence and an accelerated time to diagnosis of interventricular septal rupture than had been previously reported. Today, 0.2% of the population experiences this complication. Early reperfusion therapy may prevent the extensive myocardial necrosis that is associated with ventricular rupture.15 Patients generally have a meantime of 1 day from infarction to development of a ventricular septal defect (VSD). Many have postulated that this acceleration of rupture may be because of thrombolysis causing hemorrhage during the “lytic state,” so that if a ventricular septal rupture occurs, its time course may be accelerated.15 In the reperfusion era, advanced age, anterior infarct location, female sex, and no current smoking were found to be the most potent predictors of a VSD.15
PATHOPHYSIOLOGY
The pathophysiology of interventricular septal rupture is similar to that of free wall rupture. Without reperfusion, coagulation necrosis develops within the first 3 to 5 days. The septum that becomes necrotic is infiltrated by neutrophils, which release lytic enzymes and thus disintegrate the necrotic myocardium.16 A transmural septal infarction underlies rupture of the interventricular septum, with the tear ranging in size from millimeters to centimeters.14
The ventricular septum is very vascular. The rarity of septal rupture and the variable infarct location relate to the fact that the interventricular septum has a dual blood supply. The anterior two-thirds is supplied by the left anterior descending (LAD)
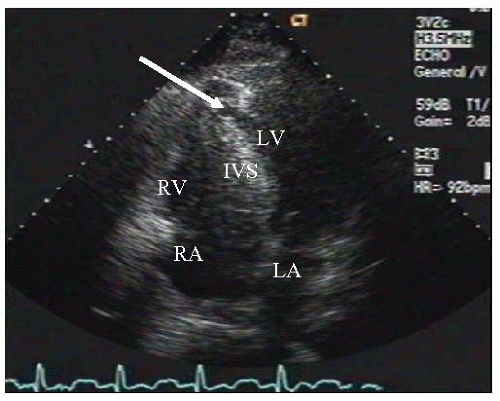
coronary artery and its branches. The posterior one-third is supplied by branches of the posterior descending artery, which come from the right coronary or the left circumflex artery, depending on the dominance of the circulation.17 Studies have conflicted in determining the artery that is predominantly responsible for septal rupture, but anterior MI is most frequently followed by inferior infarction. Similar to free wall rupture, interventricular septal rupture occurs most frequently with a first MI when there is less likely to be collateral blood flow. In this setting, with an abrupt cessation of flow in the infarct-related artery, no collateral flow exists to support the infracted zone, thereby making the septum prone to rupture (Figure 10.2).
coronary artery and its branches. The posterior one-third is supplied by branches of the posterior descending artery, which come from the right coronary or the left circumflex artery, depending on the dominance of the circulation.17 Studies have conflicted in determining the artery that is predominantly responsible for septal rupture, but anterior MI is most frequently followed by inferior infarction. Similar to free wall rupture, interventricular septal rupture occurs most frequently with a first MI when there is less likely to be collateral blood flow. In this setting, with an abrupt cessation of flow in the infarct-related artery, no collateral flow exists to support the infracted zone, thereby making the septum prone to rupture (Figure 10.2).
CLINICAL PRESENTATION
Whether in the pre-reperfusion era or post-, it is generally agreed upon that the clinical presentation and examination of a patient with a ventricular septal rupture has remained constant. Symptoms of rupture include shortness of breath, chest pain, and signs of low cardiac output and shock.16 In 1934, Sager proposed a set of clinical criteria for suspecting a ruptured septum. The sudden onset of a systolic murmur, often accompanied by a thrill, in a patient with rapid hemodynamic decompensation is highly suggestive of a ruptured interventricular septum.18 The rupture produces a harsh, loud, holosystolic murmur along the left sternal border, radiating toward the base, apex, and right parasternal area with a palpable thrill present in half of patients. Compared with acute mitral regurgitation (MR), initially there is often right ventricular failure and absence of severe pulmonary edema before left ventricular failure ensues. As the disease progresses complete biventricular failure invariably occurs.16
In the setting of cardiogenic shock, the thrill and murmur may be difficult to appreciate because turbulent flow across the defect is reduced. Pulmonary hypertension may cause the pulmonic component of the second heart sound to be accentuated. Right and left ventricular S3 gallops are often heard. Tricuspid regurgitation may be present and biventricular failure generally occurs within hours to days.16
TREATMENT
In the correct clinical setting, immediate echocardiogram is crucial to confirm the diagnosis. After the diagnosis is made, medical therapy may consist of mechanical support with an IABP, afterload reduction, diuretics, and inotropic agents.16 Prompt surgical intervention is essential as most patients have a rapid deterioration and die. It was previously believed that shortly after an AMI, the myocardium was too fragile for safe repair of the rupture. A waiting period of 3 to 6 weeks, to allow the margins of the infracted muscle to develop a firm scar to facilitate surgical repair, was standard before surgical intervention.19 Medical therapy carries close to a 100% mortality. Although surgical therapy results are poor as well, about 13% survival, it is still considered the primary therapy.20




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree