Fig. 11.1
Extracorporeal membrane oxygenation . This is a portable bedside cardiopulmonary bypass circuit, consisting of a pump head and oxygenator, with standard pressure and bubble monitoring alarms. The system provides circulatory support and respiratory support simultaneously
The use of ECMO continues to be a mainstay for short-term support of neonates, infants, and older children with acute hemodynamic compromise secondary to heart failure. It is frequently used to assist the pediatric patient with postoperative heart failure to support the circulation during recovery and has been invaluable in achieving improved surgical outcomes for the repair of complex congenital cardiac diseases [18, 19]. It can be rapidly deployed at the bedside or in the operating room and provides effective cardiopulmonary support for urgent and emergent conditions where either heart or lung support is needed. ECMO has been successfully used as a temporary support for 1–2 weeks for this population but is generally not considered suited for long-term support. In heart failure patients requiring heart transplantation, ECMO is still associated with a very high morbidity and mortality as a bridge to transplant , and survival to transplantation using long-term support with ECMO alone has been as low as 50 % [14]. Superior results have been achieved with long-term VADs [20].
Centrifugal pumps were among the earliest designs of successful pumps for pediatric cardiac support. These pumps utilize a spindle/bearing design to directly turn the spinning rotor. These early centrifugal pumps were frequently used for pediatric patient support for postcardiotomy failure and bridge to transplant when pulmonary oxygenation was adequate. In general, these pumps can be managed with lower anticoagulation requirements than an ECMO circuit. This direct drive method , however, has been implicated in a higher incidence of hemolysis and thrombus formation. A new generation of centrifugal pumps is now available that eliminates the central drive shaft/bearing. Each of these new generation pumps can generate in excess of 9 L/min of blood flow with very low priming volumes. Two of the most common examples of these pumps are the Jostra Rotaflow ® (Maquet, Wayne, NJ, USA; Fig. 11.2) and the CentriMag ® (Levitronix, Waltham, MA, USA). The Jostra Rotaflow ® (32-mL prime) is a magnetically stabilized system on a mono-pivot with a sapphire bearing. The CentriMag utilizes a magnetically levitated (maglev) impeller to avoid the central drive shaft; currently there are two sizes available that can be utilized with the same pump console depending on the patient size—adult CentriMag (32 mL prime) and pediatric CentriMag (14-mL prime). Early clinical experiences have shown that these newer-generation pumps tend to be more durable and less prone to thrombus and hemolysis than the previous centrifugal pumps. A newer pump, the PediMag (Thoratec Corp., Pleasanton, CA, USA), is a 2.5 L/min centrifugal pump that is approved in the United States for 6-h use and is only designed for short-term support for up to 7 days.


Fig. 11.2
Jostra Rotaflow® is an example of a new generation of centrifugal pumps that eliminates the central drive shaft/bearing
Axial-flow pumps for short-term use are currently limited to the catheter-based devices. The Abiomed Impella 2.5 (Abiomed Inc., Danvers, MA, USA) is a catheter-based micro-axial-flow device that is inserted intravascularly into the left ventricle in a retrograde manner across the aortic valve and can produce flows up to 2.5 L/min. It is currently approved for short-term support up to 7 days and has been used successfully in the older pediatric patients. These devices can be placed percutaneously using echocardiographic guidance.
11.6 Long-Term Support
When mechanical support is anticipated for longer than 2–3 weeks as bridge to recovery or (more often) as bridge to transplant, then devices capable of greater durability and low-risk long-term support are preferred. Short-term devices such as ECMO or centrifugal pumps may be used initially as a bridge to implantation of a longer-term device (bridge to a bridge).
A variety of options are available for long-term mechanical support of the pediatric patient. In contrast to adults, all currently available devices that are specifically designed for smaller children are paracorporeal, which does not allow patients to be discharged to home with the device. Newer and smaller devices currently under investigation are being designed for intracorporeal implantation with either an exiting driveline or possibly a transcutaneous energy transmission (TET) source. These devices would potentially allow pediatric patients to be discharged to their homes during long-term mechanical support, thus as a bridge to transplantation.
The Berlin Heart Excor pediatric VAD (Berlin Heart, Berlin, Germany) was the first commercially available ventricular assist system designed specifically for the pediatric population, i.e., with miniaturized pumps and special cannula. It is a paracorporeally placed, air-driven pump in a polyurethane, translucent, semirigid chamber that houses the blood-contacting membrane. The blood pumps come in 10-, 12-, 15-, 25-, or 30-mL stroke volumes and offer support options for pediatric patients as small as 2.5 kg (Fig. 11.3). These pumps are driven by a pulsatile electro-pneumonic system and can be used as a right, left, or using two as a biventricular system. The pumps can be easily exchanged for issues of thrombus formation or to increase the size of the pump in response to a growing child on long-term support. Today, the Berlin Heart Excor is the most commonly used pediatric mechanical support VAD and was approved by the FDA in December 2011. It has as high as a 90 % survival as bridge to transplant in pediatric patients and has proven more effective than ECMO for bridge to transplant support [21]. However, the incidence of neurologic dysfunction and complications from embolism has been reported to be as high as 29 %. To date, there have been over 1200 implants in pediatric patients in over 34 countries worldwide [22].
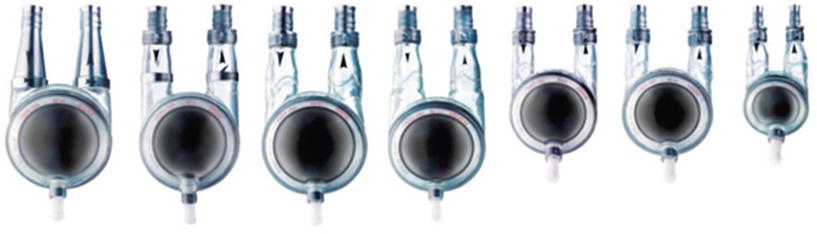

Fig. 11.3
Berlin Heart Excor pediatric ventricular assist device . This was the first commercially available ventricular assist system designed specifically for the pediatric population (with miniaturized pumps and special cannula)
The HeartMate II (Thoratec Corp.) is an LVAD axial-flow device that can generate flows of up to 10 L/min, weighs 176 g, and measures 40 mm in diameter (Fig. 11.4). Early clinical trials show very effective hemodynamic support with improved patient functional status and quality of life. The HeartMate II was FDA approved in January 2010 for use as destination therapy in adults with heart failure. Today, over 4000 HeartMate II devices have been implanted in adults and children worldwide, making it the most frequently used and successful VAD in the current era [23, 24]. The pump was designed for patients greater than 1.1 m2 BSA, but it has been used to support patients with BSAs less than this. Yet, because it is an implantable pump designed primarily for use in adults, it has limited use in smaller pediatric patients.
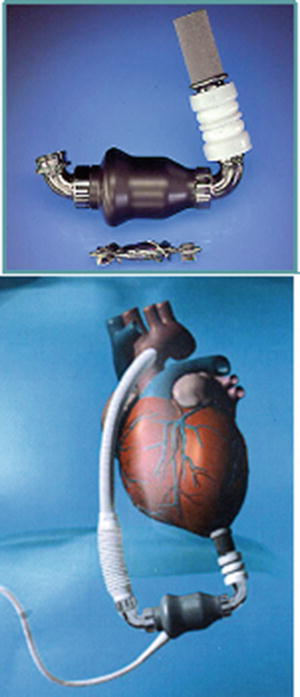

Fig. 11.4
HeartMate II left ventricular assist device . This axial-flow device can generate flows of up to 10 L/min and was FDA approved in January 2010 for use as destination therapy in adults with heart failure
The Heartware HVAD™ (HeartWare Ltd. Sydney, Australia) pump is an intracorporeal centrifugal maglev device that is implanted in the left ventricular apex and has been successfully used in smaller patients. At the core of the HVAD pump platform is a proprietary hybrid system for suspending the impeller (or rotor), the only moving part within the pump. Once power is applied to the device and the impeller begins to rotate, there are no points of mechanical contact within the HVAD pump, effectively ensuring a contactless system. The elimination of mechanical bearings is expected to lead to longer-term device reliability. The suspended impeller is designed to provide optimal blood flow paths through the system and to reduce red blood cell damage. The HVAD is accredited for patients with a BSA >1.2 cm2; due to its small size, this pump has more recently been used in children. To date, over 160 implants have been reported worldwide in the pediatric age groups.
The Medos HIA VAD (Medos Medizintechnik GmbH, Stolberg, Germany) is similar to the Berlin Heart VAD. It is a pulsatile VAD, pneumatically driven with a pump chamber made of polyurethane that is available in various sizes from 10 to 80 mL so it can be used in pediatric patients as small as 3.0 kg. It can also be used in a right, left, or biventricular configuration. It still has limited availability in the United States and does not have FDA approval at this time.
Thoratec Corp. also offers both an intracorporeal (Thoratec ® IVAD) and paracorporeal (Thoratec ® PVAD) VAD system. They both allow the option of right, left, or biventricular support and are approved for use as a bridge to transplantation and for support in the setting of postcardiotomy shock. They provide pulsatile flow with a 65-mL blood chamber, and unidirectional flow is achieved with tilting disk mechanical valves. The paracorporeal placement of the PVAD allows use in patients with a BSA of <1.5 m2 and has been used successfully in patients < 0.8 m2.
The SynCardia Total Artificial Heart (TAH) (SynCardia, Inc., Phoenix, AZ, USA) is a pneumatically driven pulsatile device with two 70-mL polyurethane pumps that generate up to 9.0 L/min of flow and are designed for total biventricular support. It is FDA approved for bridge to transplant and has also received compassionate use approval as destination therapy. A portable driver was introduced in 2011 and has greatly facilitated the mobility of patients following device implant. Unlike other assist devices, the TAH actually replaces the failing ventricles and is implanted using the atrial chambers to fill the pumps through prosthetic valves. Long-term survival has been reported with the device. Recent use in single ventricle failing Fontan patients as bridge to transplant was reported [25]. While the 70-mL pump device is only used in adult-sized patients, a smaller 50-mL pump device for use in older children with a BSA as low as 1.22 m2 is scheduled to be introduced in 2015.
11.7 Challenges of Designing Pediatric Ventricular Assist Devices
The challenge of designing pediatric VADs stems, in large part, from the issues of smaller-sized patients with limited chest capacity, rapid growth potential, and the fragility of the cardiovascular structures that may be involved with implantation. The challenge of miniaturization of devices to be used or implanted in the pediatric population has impeded the advancement and use of VADs in these patients until recently. In addition to the limited space in the thoracic cavity of smaller and younger children, there is also the issue of small caliber vasculature, small circulating blood volume, and small cardiac chambers. Interfacing a VAD with the cardiovascular system of a child must allow for these factors. In addition, the fragility of the cerebral vasculature of neonates and younger children presents a concern that damage and hemorrhage may occur due to elevated systolic pressure generated by a device. Furthermore, the issues of growth in these patients and the sizing of devices for potentially rapidly growing children add yet another challenging dimension to these patients, not encountered in the adults.
Anticoagulation is also more difficult to manage in these young patients, and medications such as warfarin (Coumadin) can be difficult to maintain in a therapeutic range without significant fluctuations. In part this may be due to the less mature coagulation cascade systems present in younger children or variations in diet and better compliance in older age groups. It follows that the design of future pediatric devices needs to be associated with reduced risks of thromboembolism in the face of fluctuating levels of anticoagulation. The issues of mobility and ambulation are also concerns in this young patient population, with the possibility of kinking of drivelines and dislodgement of devices occurring if not securely implanted and anchored in an active and mobile child. Long-term support and the longevity of the pumps and materials over time are yet another challenge with pediatric devices. While totally implantable devices are desirable in some respects, issues like upsizing of pumps for growth and removal for thrombus, infection, or device failure make implantable devices more problematic for long-term support.
The variable anatomies of congenital heart defects (either before or after surgical intervention) and the interface of devices with abnormal anatomies can be surgically and technically challenging. More specifically, the presence of hypoplastic cardiac or vascular structures, residual shunts, insufficient valves, dextrocardia or heterotaxy, and abnormal venous return related to previous Glenn or Fontan cavo-pulmonary reconstructions all pose significant surgical challenges in this group of patients. Further, the presence of aortopulmonary shunts, either from previous palliative surgery or development related to the congenital defect, can create altered flow patterns during mechanical support by devices and result in pulmonary overcirculation.
It should be noted that the use of long-term devices in the single-ventricle patient population represents one of the greatest challenges in supporting patients with congenital heart disease. Given the anatomic complexities of the cavo-pulmonary connection and various modes of Fontan failure, any single configuration for mechanically supporting this circulation will probably not be effective for all patients. Research into the design and development of such devices and their interface with this Fontan circulation is increasing, to meet the growing population of patients with failing Fontans [26–28].
ECMO support for single-ventricle patients also poses particular challenges. Management of the systemic-to-pulmonary artery shunts must be determined based on the indication for ECMO support, systemic ventricular function, and flow characteristics of the ECMO circuit. In patients with an aortopulmonary shunt who are unable to handle any pulmonary overcirculation or cardiac volume overload, occlusion of a shunt may be indicated while on ECMO support [29]. The presence of a Glenn or Fontan reconstruction may also require a strategy of bi-caval cannulation for adequate venous decompression. Outcomes with ECMO and other devices in this patient population have been substantially lower than any other group [30]. A recent study from the ELSO registry on VAD use in pediatric patients showed only a 27 % survival in the single-ventricle cohort [31]. For more details relative to these congenital defects, the reader is referred to Chap. 10.
11.8 Future Pediatric Devices
The future development of pediatric devices will include the continued miniaturization of technologies, the development of alternate energy strategies, and the continued advancements in the designs, such as: (1) maglev technology, (2) the use of transcutaneous energy sources, and (3) laminar flow devices that are completely implantable without the need for an external driveline. Importantly, several devices are currently in clinical investigation or animal research trials that demonstrate many of these evolving trends in pediatric VADs.
HeartWare MVAD pump (MVAD, HeartWare®, Inc., Miramar, FL, USA) is a continuous axial-flow pump, approximately one-third the size of the HVAD® pump (Fig. 11.5). The MVAD pump is based on the same proprietary contactless impeller suspension technology used in the HVAD pump, with its single moving part held in place through a combination of passive-magnetic and hydrodynamic forces. The MVAD pump was designed to support a wide range of flows to enable both full and partial support capabilities. Additionally, it is expected that MVAD’s small size and wide range of flows will enable support of smaller patients and those with right ventricular failure. A key focus is the development of a fully implantable system based on transcutaneous energy transfer technology. This technology enables a fully implanted battery pack to be periodically recharged using inductive coupling across the skin. This will allow implantation of the complete system, including batteries and controllers, and will eliminate the current need for a percutaneous driveline to a permanently connected controller. The HeartWare MVAD is scheduled to be available in the very near future.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


