VISCERAL ANEURYSMS AND PSEUDO ANEURYSMS
Visceral artery aneurysms and pseudoaneurysms are defined as localized arterial dilatations ≥1.5 times the expected diameter of the normal artery. These aneurysms include dilations found in the splenic, celiac, hepatic, superior mesenteric, inferior mesenteric, and renal arteries. Although the true prevalence is not known, autopsy reports suggest that visceral artery aneurysms and pseudoaneurysms have a prevalence ranging from 0.1% to 2% (1). The increasing use of ultrasound and computed tomography has concomitantly increased the incidence of the incidental finding of visceral artery aneurysms. In addition, increased instrumentation of the pancreaticobiliary tract and nonoperative hepatic trauma management has also increased the incidence and detection of visceral pseudoaneurysms (2). Although rare, rupture of these aneurysms and pseudoaneurysms result in mortality rates ranging from 21% to 100% (1).
Historically, management of visceral artery aneurysms consisted of either serial observation, open surgical ligation, or aneurysmectomy (3). However, since the initial reported cases of endovascular repair, endovascular techniques have emerged as a safe and efficacious alternative treatment to open surgical repair. This improved efficacy and decreased morbidity have resulted in preferential utilization of endovascular methods to manage visceral arteries aneurysms and pseudoaneurysms (4) by many vascular surgery and interventional groups. Because the natural history of the visceral aneurysms and pseudoaneurysms is unclear, endovascular repair is particularly attractive in that they allow physicians to avoid the morbidity associated with a laparotomy. Endovascular techniques are continually evolving, but the present mainstays at the time of publication include the instillation of liquid embolization, coil embolization, and, more recently, stent graft exclusion with both balloon-expandable and selfexpanding covered stent technology (1–6).
This chapter will summarize the techniques and materials available to interventionalists to exclude visceral aneurysms and pseudoaneurysms. Special emphasis will be placed upon the techniques utilized by our vascular surgery group. Moreover, the chapter will discuss patient selection, periprocedural management and outcomes of the patients, as well as a discussion of intra- and postprocedural complications. The focus of this chapter will be upon splenic and renal artery aneurysms, with brief mention of hepatic and superior mesenteric artery aneurysms and pseudoaneurysms.
ANATOMIC CONSIDERATIONS
The branches of the abdominal aorta are among the most variable in the human body, with some authors stating that less than 25% of the splanchnic vessels studied follow the “classic” course (7). The renal vessels are more consistent, though there are several variations that are important to recognize. Although multidetector computed tomography (CT) with three-dimensional reconstructions will help identify most anomalous anatomy, knowledge of the common variations is vital to accurately localizing and approaching target vessels, and avoiding technical misadventure.
The celiac trunk is present in approximately 99% of patients (7,8). The “classic” description of the left gastric, splenic, and proper hepatic arteries arising from a common trunk is found in 60% to 89% based on cadaveric dissections and review of CT angiographic data (8). The most common variant is the “gastrosplenic” trunk, which occurs in 5% to 8% of patients and is composed of a common trunk for the left gastric and splenic arteries, and a separate hepatic artery that arises independently from the aorta. Other uncommon variants include the hepatosplenic and hepatogastric trunks. Most rarely, the superior mesenteric artery will arise from the celiac trunk (7,8). An important landmark for the origin of the celiac trunk is the top of the L1 vertebral body (7).
The normal hepatic arterial anatomy is present in 70% to 80% of the time (7,9). The most frequent anomaly encountered in the hepatic arterial tree is an aberrant right hepatic artery, arising from the superior mesenteric artery in 12% to 20% of individuals. The aberrant left hepatic artery arising from the left gastric artery is the next most common variant, occurring in approximately 3% of individuals. With respect to the origin of the common hepatic artery, there are two anomalies that the interventionalist should be aware of: (1) an absent common hepatic artery, occurring in approximately 12% of individuals and (2) an anomalous common hepatic artery, arising as a branch of the superior mesenteric artery, occurring in approximately 4% of individuals (7,9).
The splenic artery usually arises from the celiac axis just distal to the origin of the left gastric artery and follows a serpiginous, retroperitoneal path along the dorsal border of the pancreas (1,7,8). Along the way, the splenic artery will provide the dorsal pancreatic branch and several smaller pancreatic branches, before dividing into four to five branches at the splenic hilum. After providing the splenic hilar branches, the splenic artery will then give off the short gastric arteries within the gastrosplenic ligament. The terminal branch of the splenic artery becomes the left gastroepiploic artery, within the gastrocolic ligament (1,7).
The last major splanchnic vessel to be discussed is the superior mesenteric artery. The superior mesenteric artery most frequently arises from the aorta at the level of the bottom of the L1 vertebra (7). Variations in this vessel mostly occur in the colic branches, in the form of either absent colic branches, or accessory colic branches. Otherwise, anomalous origin of the right hepatic artery or the common hepatic artery from the superior mesenteric artery also frequently occurs (7,9).
The renal arteries typically arise within 2 cm of the L1–L2 disc space, with the origin of the right renal artery being typically higher than the left. In three quarters of cases, there is a single hilar vessel supplying each kidney (7,10). Supernumerary arteries are classified as either hilar or polar accessory vessels, with a combined frequency of 24% (10). Bilateral supernumerary renal vessels occur in 5% of individuals. Pre-hilar branching of the renal artery is frequent, occurring in 8-13% of individuals (7,10).
EPIDEMIOLOGY, CLINICAL PRESENTATIONS, AND INDICATIONS FOR INTERVENTION
Splenic Artery Aneurysms
Splenic artery aneurysms have the highest prevalence among visceral artery aneurysms, comprising 60% to 75% of all visceral artery aneurysms (1–3,11). The majority (>70%) of splenic artery aneurysms are true aneurysms. Most patients (up to 80%) are female and present in their early sixties (1,11). Presentation with rupture occurs infrequently (5%), with over 90% of patients presenting with the splenic artery aneurysm as an asymptomatic finding on plain radiograph, CT, or arteriogram (1–3,11). Patients with splenic artery aneurysms are more frequently multiparous (1,11), hypertensive, and obese (11). Other frequent comorbidities include coronary artery disease, hypercholesterolemia, peptic ulcer disease, and cirrhosis. Multiple visceral artery aneurysms can occur in up to one third of patients (1). The most frequent location of visceral aneurysms associated with splenic artery aneurysms include the renal artery (7.4%) and extrahepatic hepatic artery (2.3%) (11). Although arteriography remains the gold standard for diagnosis, CT angiography has evolved sufficiently to allow the diagnosis to be made by CT angiogram alone, with arteriography reserved for patients undergoing a planned repair of the aneurysm (1).
The largest series of splenic artery aneurysms was published by Abbas et al. (11) who reported a retrospective single-center experience of 217 consecutive splenic artery aneurysms over 18 years. The vast majority (95%) of splenic artery aneurysms were solitary. Ruptured splenic artery aneurysms were larger, particularly in males, with a mean diameter at rupture of 3.1 cm, versus 2.2 cm for nonruptured. The mean aneurysm diameter at the time of repair for elective aneurysms was 2.7 cm. Mean aneurysm growth rate was 0.06 cm/year. Mortality for a ruptured aneurysm was 20% in their series, whereas the mortality after an electively repaired splenic artery aneurysm was 5.1%. Among pregnant patients, the rates of maternal mortality (>20%) and fetal mortality (>90%) were extraordinarily high in those with rupture. Because of the high mortality associated with rupture, intervention is recommended for all patients presenting with symptomatic aneurysms, those women with aneurysms discovered during pregnancy or of childbearing age, and those discovered incidentally in a patient undergoing evaluation for liver transplantation (1,11). Abbas et al. (11) recommend treatment for splenic artery aneurysms greater than 2 cm in patients who are “reasonable” operative candidates with a ≥2 year life expectancy.
Operative options include ligation (open or laparoscopic) or resection and reanastomosis (Figs. 20.1 and 20.2). Both operative techniques may or may not include a splenectomy or distal pancreatectomy (1,11). Endovascular options include embolization (glue or coil) and/or exclusion using a covered stent (1–4,11). Although there are some that preferentially utilize endovascular methods of repair (3,4), open surgical repair is still the gold standard, with endovascular options being reserved for high-risk surgical patients (11). Moreover, some series report a high incidence of splenic and pancreatic tail infarcts with embolization of distal splenic artery aneurysms at the hilum and recommend open surgical repair for these patients (3).
Pregnancy is not a contraindication to endovascular repair; however, the risks of catheter-based intervention during pregnancy (i.e., radiation and iodinated contrast) must be weighed against the risk of conservative management. The maximum allowed fetal dose of radiation is 5 rad, with the most critical time being between 10 and 17 weeks of gestation, where central nervous system development is most prolific (12). During pregnancy, iodinated contrast does not show any adverse teratogenic effects, though there is the potential to suppress neonatal thyroid function. Therefore, it is recommended that thyroid function be evaluated during the first week of life (13). If a pregnant patient presents with a ruptured splenic artery aneurysm, however, celiotomy (incision into the abdominal cavity) is recommended so that an emergent caesarean section can be attempted (11).
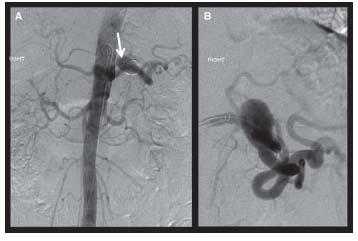
Figure 20.1 • Abdominal aortogram and selective splenic arteriography for treatment planning. A reveals a splenic artery aneurysm as demonstrated on AP abdominal aortic imaging. The white arrow is directed toward the calcified appearing aneurysm. B is a selective arteriogram of the splenic artery demonstrating the significant tortuosity that can make endovascular therapy challenging in this location.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



