For patients with descending thoracic aortic aneurysms (TAA), thoracic endografts have demonstrated a dramatic improvement in patient outcome compared with open surgical repair. Dreaded complications, such as paraplegia, have a significantly lower incidence in patients treated with endografts compared with reports from open aneurysm repair (3% vs. 15%), and procedural mortality is also significantly reduced (1,2). However, a new set of complications, largely related to arterial access issues, has been noted that are specific to the use of these endografts. Many of these complications can easily be avoided by careful patient selection, preprocedural planning, and attention to details during the procedure.
There are currently three thoracic endografts approved for use in the United States. Each device has certain unique features, specifically related to the deployment technique. Many physicians view the descending thoracic aorta as a simple, straight tube, and thus surmise that the endovascular treatment of TAA using endografts is technically less challenging than that of infrarenal abdominal aortic aneurysms (AAA). However, the thoracic aorta presents unique challenges that have resulted in a significant time lag between the availability of AAA endografts and FDA-approved thoracic endografts. This chapter summarizes the general principles for performing endovascular repair of TAA, highlighting tips to avoid common pitfalls. Technical steps specific to each unique device are not discussed in detail.
ARTERIAL ACCESS
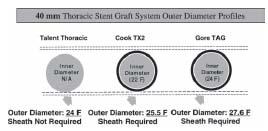
Arterial access continues to represent a major challenge for endovascular repair of TAA. This is largely based on the requirement for large diameter access sheaths to deliver large diameter devices (Fig. 19.1). The single most frequently encountered complication in thoracic stent graft trials involves the arterial access and conduit iliac vessels. Vascular trauma or thrombosis of the iliac vessels was noted in 14% of patients in the thoracic aortic graft (TAG) phase II muticenter trial, and in 6% of patients in the phase III study (1). Access complications are most commonly encountered when using a 24-Fr. sheath in patients with borderline iliac diameters. Smaller iliac diameters combined with calcification and tortuosity further increase the risk of iliac dissection or rupture. Preoperative planning should involve computed tomography (CT) scans that image caudally to the level of the common femoral artery. Both contrast and noncontrast CT scans need to be evaluated to measure the smallest common femoral and iliac artery diameter and the extent of calcification (Fig. 19.2). Tortuosity is best evaluated with 3D reconstruction of CT data or conventional angiography (Fig. 19.3). The author typically performs conventional diagnostic angiography with a marker pigtail catheter to supplement CT data.
It is important to watch the sheath move into the aorta at all times under fluoroscopy. The sheath is ideally best introduced over a stiff wire (e.g., Lunderquist or Meier). Additionally, it is important to remember that the vast majority of iliac injuries are noticed during sheath withdrawal. Hence, if excessive force is required to introduce a sheath, an iliac tear may be encountered during sheath withdrawal (Fig. 19.4). If the sheath is not easily withdrawn, it may be advisable to plan for an iliac exposure with the sheath in place or to have an adequate diameter contralateral femoral sheath (12 Fr.) and an aortic occlusion balloon (e.g., Reliant or Coda) readily available to allow for control of aortic inflow, if required. It is also important not to lose wire access as temporary tamponade of iliac injuries may sometimes be achieved by reintroducing the sheath and dilator until a definitive repair is undertaken.
Occasionally, during sheath withdrawal, an intimal flap may be noted to be adherent to the sheath. In these cases, after closure of the femoral arteriotomy, a completion pelvic angiogram using contralateral femoral or brachial access may help in the early identification of an iliac pseudoaneurysm that may result in a delayed rupture (Fig. 19.5). In the event that two or more thoracic endograft devices are required, a long introducer sheath is advisable as this prevents repeated trauma to the iliac/femoral artery during introduction of each subsequent device.
USE OF CONDUIT
As a rule, when in doubt, it is advisable to obtain arterial access through a conduit. Overall, 15% of patients in the TAG (99-01) clinical trial had a conduit to the aortoiliac segment to facilitate access. Iliac conduits are best performed through a left flank incision. The author prefers to use a left common iliac artery conduit, as the left iliac vein is less prone to injury in this location. A 10-mm Dacron graft is sewn in an end-to-side fashion (Fig. 19.6A) and brought out through a stab wound in the lower part of the abdomen (Fig. 19.6B). This allows for a straight and easy access as opposed to introducing the device directly into the common iliac artery. Clamping the distal end of conduit (Fig. 19.6B), and accessing it as though it were an artery, allows for better control of blood loss. This technique allows the physician to make an incision into the conduit only as big as the required sheath. Use of vessel loops, and/or umbilical tapes, around the conduit can also help decrease blood loss when the sheath is in place. At the completion of the procedure, the author usually converts the conduit to an iliofemoral bypass. This allows for a ready access site in the event of a need for a delayed intervention in the future.
Figure 19.1 • Large diameter introducer sheaths required for the thoracic stent grafts.
Occasionally, the common iliac artery is of inadequate caliber and a direct aortic conduit is required (Fig. 19.7). In the author’s experience, this is usually only required at the time of visceral debranching procedures or in young trauma patients with traumatic thoracic aortic pseudoaneurysms with small iliac vessels.
DEVICE INTRODUCTION AND PLACEMENT
Regardless of the location of the TAA, it is routine practice to place a stiff wire with the distal tip against the aortic valve to facilitate device delivery. Once guidewire placement has been confirmed on fluoroscopy, the operating room (OR) table is locked in position with the location of the back end of the access wire marked on the table. Thoracic endografts are available in 20-cm lengths and, depending on the size of the field of view of the imaging system, cannot always be captured within a single fluoroscopic image. For example, if the distal end of a 20-cm graft needs to be deployed immediately proximal to the celiac axis, the proximal end of the graft may not be visualized during this maneuver. Hence, a fixed wire position avoids a proximal “unseen” complication that may result from inadvertent distal migration of the guidewire. Loss of wire from the proximal end of the graft may result in the graft folding within the aneurysm and subsequent inability to achieve wire access beyond the graft. Figure 19.8 demonstrates the first portion of a thoracic endograft that has been deployed; it is obvious that loss of guidewire access at this point would result in the proximal end of the stent graft folding within the aneurysm. It is also important to fluoroscopically visualize the leading point of the graft at all times. At no point is it recommended that grafts be moved without visualizing the leading edge.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree