■ In situ replacement: Low-grade infections without sepsis or invasive infection and those with distal occlusive disease may be best treated with in situ graft replacement.
■ Graft replacement material
■ Graft material should be considered prior to surgery to ensure availability.
■ Potential options for graft material include the following:
■ Autogenous vein (saphenous vein, cephalic vein, basilic vein, superficial femoral vein [SFV])
■ Cryopreserved tissue (aorto-iliac-femoral artery, femoral vein, saphenous vein)
■ Prosthetic graft (rifampin-soaked Dacron™, polytetrafluoroethylene [PTFE])
■ Consider the need for wound coverage:
■ Debridement of an infected groin wound may result in a large defect that either cannot be covered or closed. Muscle flaps can provide coverage of healthy well-vascularized tissue to protect the repair.
■ Small to medium defects can be covered with a sartorius muscle flap, which is divided from its attachment to the anterior superior iliac spine and mobilized medially to cover the wound.
■ A pedicled flap from the leg or abdominal wall may be required for larger wounds. These flaps include rectus femoris, rectus abdominis, tensor fasciae latae, or gracilis.
TECHNIQUES
GENERAL CONSIDERATIONS
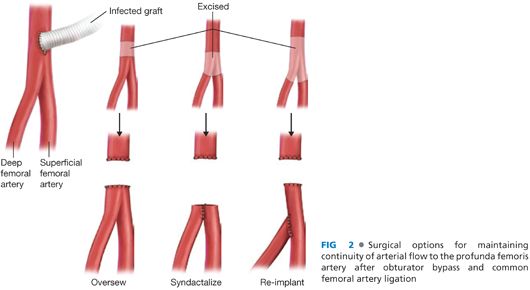
■ It is desirable when considering an extraanatomic reconstruction to revascularize before excising the infected graft. This can be accomplished with a bypass and tunnel performed across clean tissue planes. Once the bypass is completed and wounds closed, the groin can be explored and the infected graft removed. With this approach, the continuity between the superficial femoral and deep femoral arteries should be maintained by either oversewing the distal common femoral artery or anastomosing the profunda femoris artery (PFA) to the superficial femoral artery with proximal ligation (FIG 2).
■ Debridement of the infected site must include removal of infected or necrotic tissue and complete excision of the anastomosis. Dissection may be aided by lack of incorporation of the infected graft but also may prove challenging from extensive scarring in the reoperative field. Sharp dissection techniques are critical to minimizing the risk of inadvertent injury to vessels or adjacent structures.
■ It is important to send cultures of the perigraft fluid, tissue, and graft.1 Instructions should be given to the microbiology lab to perform sonication of the graft to separate biofilm from graft and maximize the bacteriology yield.
OBTURATOR BYPASS
■ Using the obturator foramen may be a useful approach for bypassing an infected groin through a sterile field.2 A reinforced PTFE graft is best suited for this technique and can be used if sepsis has been controlled and the bypass can be performed without violating the infected field.
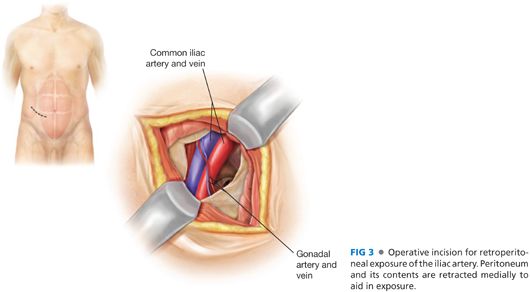
■ The proximal anastomosis can be performed to the common iliac artery, external iliac artery (ipsilateral or contralateral), or previous graft if not infected (FIG 3). Exposure can be obtained through a standard retroperitoneal incision, by dividing the external and internal oblique and transversus abdominis muscles, identifying the preperitoneal space, and retracting the peritoneum medially with blunt dissection techniques. The obturator foramen is just posterior to the anterior ramus of the pelvis, although it may not be easily palpated due to the overlying obturator membrane.
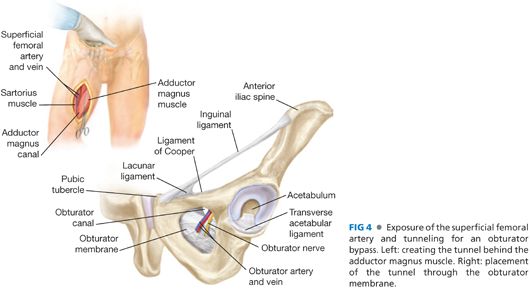
■ Distal anastomosis can be performed to the distal superficial femoral artery, the midportion of the PFA (see the following section, lateral approach to the Lateral Profunda Femoris Artery Exposure), or the popliteal artery. During this dissection, the adductor longus and magnus can be identified with the leg abducted and externally rotated. The tunnel will be placed deep to these muscles, which insert on the external surface of the obturator foramen.
■ The tunnel should be performed in a cranial direction with a long aortic clamp or tunneling instrument (FIG 4). The instrument is passed deep to the adductor magnus while a hand is placed over the obturator foramen from the retroperitoneal incision. The instrument can be directed through the obturator foramen. The tunnel should be made through the lateral portion of the obturator foramen to avoid the obturator artery and nerve, which traverse anteromedially.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


