Chapter 11
Management of Peripheral Arterial Disease
Introduction
The aim of this chapter is to focus on the complex diagnostic and therapeutic stragegies that are necessary to inhibit cardiovascual events, amputations, and life loss. The following case may enlighten the various problems.
A 61-year-old female patient with type 2 diabetes mellitus presents at the outpatient department with a reduced walking distance of 100 meters due to pain in the right calf, corresponding to peripheral arterial disease (PAD) in stage IIb. The PAD of the patient was already treated in the past with two stents in the right proximal superficial femoral artery. The patient is obese, has hypertension, and suffers from diabetic polyneuropathy and chronic venous insufficiency grade 1. Smoking history shows 80 pack-years (of cigarettes). In addition to her PAD, the patient has undergone cardiac bypass grafting five years ago, had already had a minor stroke, and is on anticoagulant therapy for atrial fibrillation.
Patients with type 2 diabetes have a substantially higher risk of mortality, primarily from cardiovascular disease, than the general population [1]. PAD, referring to atherosclerotic occlusive disease of the lower limb arteries is a common, debilitating complication that correlates with cardiovascular disease mortality [2].
Diabetes is a significant independent risk factor for PAD (odds ratio of 2–3) [3], together with hypertension, cardiovascular disease, hyperlipidemia, smoking, and obesity [3, 4]. The prevalence of PAD in patients with type 2 diabetes has been estimated at 23.5% in a UK population [5], and is strongly dependent on the duration of diabetes [6, 7]. Compared with men without diabetes, the adjusted relative risk of PAD among men with diabetes increased from 1.39 with diabetes duration of 1–5 years’ to 4.53 for diabetes of >25 years’ duration [7]. Remarkably, a very high prevalence (71%) of PAD was recently reported in 1,462 elderly patients with diabetes (>70 years) in Spain as evaluated by a pathological ABI (ankle-brachial index) [8].
Diabetes Is an Important Risk Factor for Lower-Extremity Amputation
Amputation of the lower limb is one of the most feared adverse health outcomes among patients with diabetes. The result is frequently devastating in terms of social functioning and mood. Amputations are usually preceded by a foot ulcer and the most important factors predicting a poor outcome of these ulcers are the extent of tissue loss, infection, peripheral arterial disease (PAD), and comorbidity [2–4]. The reasons for a major amputation are limited; the most frequent reasons are critical limb ischemia with rest pain or progressive infection in a leg that cannot be successfully revascularized [5]. Sometimes an immediate amputation is performed because of life-threatening infection or infection with massive tissue loss. In addition, a minor amputation is frequently performed for a forefoot abscess, osteomyelitis, or gangrene of a toe. If other options are exhausted or undesirable, amputation can therefore be a treatment and not a failure.
A prospective study identifying risk factors for lower-extremity amputation (LEA) of 776 US veterans in Seattle shows that peripheral sensory neuropathy, PAD, foot ulcers (particularly if they appear on the same side as the eventual LEA), former amputation, and treatment with insulin are independent risk factors for LEA in patients with diabetes [6]. A recent meta-analysis [7] including 94,640 participants and 1,227 LEA cases reported in 14 studies demonstrated a substantial increase in the risk of LEA associated with glycemia in individuals with diabetes. The overall risk reduction (RR) for LEA was 1.26 (95% CI 1.16–1.36) for each percentage point increase in HbA1c. The estimated RR was 1.44 (95% CI 1.25–1.65) for type 2 diabetes and 1.18 (95% CI 1.02–1.38) for type 1 diabetes. Remarkably, the risk for LEA was not different in patients with diabetes duration < or >10 years.
The presence of PAD is a very important predictor of amputation in patients presenting with the diabetic foot syndrome (DFS). In a recent study [3] investigating a large cohort of diabetic foot patients (n = 1,088) treated at centers of excellence in 10 different European countries, the major amputation rate among patients with PAD during a 12-month follow-up was 8%, compared to only 2% among patients without PAD (p < 0.001). In two recent studies [4, 9], severe PAD (ankle pressure <50 mmHg or toe pressure <30 mmHg) was a predictor of increased major amputation risk in diabetic patients with neuroischemic or ischemic foot ulcers.
Poor Prognosis of Patients with Diabetes after Amputation
A Scottish study showed that after LEA diabetic subjects had a 55% greater risk of death than those without diabetes [10]. Median time to death (Figure 11.1) was 27.2 months with diabetes versus 46.7 months without diabetes (p < 0.01) and survival rate 10 years after amputation was 22.9% in nondiabetic patients but only 8.4% in diabetic patients (p = 0.0007). In a recent 10-year follow-up study [11], a first major amputation occured in 38 of 257 (15.4%) patients with diabetic foot ulcers. All but one of these patients had evidence of PAD at inclusion in the study, and 51.4% had severe PAD (ABI <0.4). Cumulative mortalities at year 1, 3, 5, and 10 were 15.4, 33.1, 45.8, and 70.4%, respectively. Significant predictors for death were age, male sex, chronic renal insufficiency, dialysis, and PAD. Thus, although long-term limb salvage in this current series of diabetic foot patients is favorable, long-term survival remains poor, especially among patients with PAD or renal insufficiency.
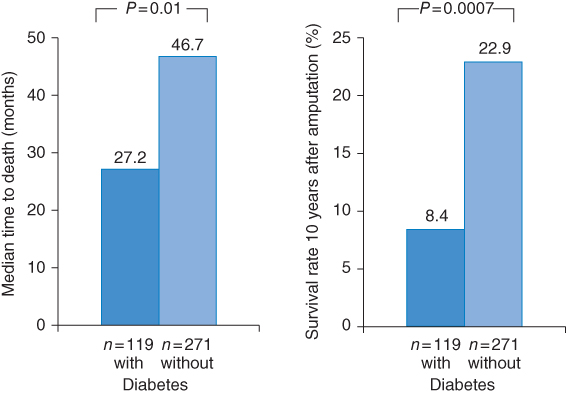
Figure 11.1 Mortality rates of patients with and without diabetes mellitus after amputation. (Source: Data from Schofield 2006 [10].)
Decrease in the Amputation Rates in Patients with Diabetes
For years, the diabetic foot syndrome did not achieve the promise of the 1989 St. Vincence Declaration. In contrast to asthonishing advances in the treatment of diabetic nephropathy, retinopathy, and coronary events, for years the amputation rates remained high.
In very recent years (after 2000) several studies in Scotland [12] and the USA [13, 14] showed a dramatic decrease in amputation rates in patients with diabetes mellitus. The incidence of major amputations in Tayside, Scotland [12] decreased from 5.1 [95% CI 3.8–6.4] to 2.9 (95% CI 1.9–3.8) per 1,000 patients with diabetes (p < 0.05) over a seven-year period (2000–06). From 1996 to 2008, the age-adjusted nontraumatic LEA rates among US residents aged ≥40 years [14] decreased in diabetic patients by 67% (p < 0.001; Figure 11.2). Despite a much greater decrease in LEA rates, the age-adjusted LEA rate in the diabetic population was still about eight times the rate of the nondiabetic population in 2008 (3.9 vs. 0.5 per 1,000 persons). A five-year follow-up of veterans’ health administration healthcare system users with diabetes and without prior amputations in 2000 (n = 405,580) and in 2004 (n = 739,377) showed that the age- and sex-standardized LEA rates decreased by 34% during the five-year period from 2000 to 2005 [14]. Of major amputations, below-knee rates decreased by 19% and above-knee decreased by 49% (Figure 11.3).
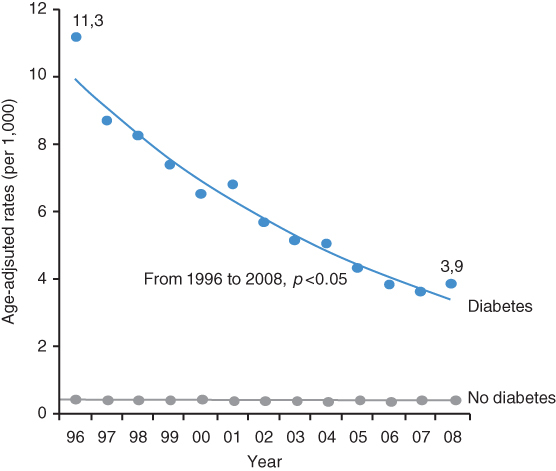
Figure 11.2 Nontraumatic amputations of lower extremities, age-adjusted per 1,000 patients after 40 years of age from 1996–2008 in the USA. (Source: Data from Li 2012 [14].)
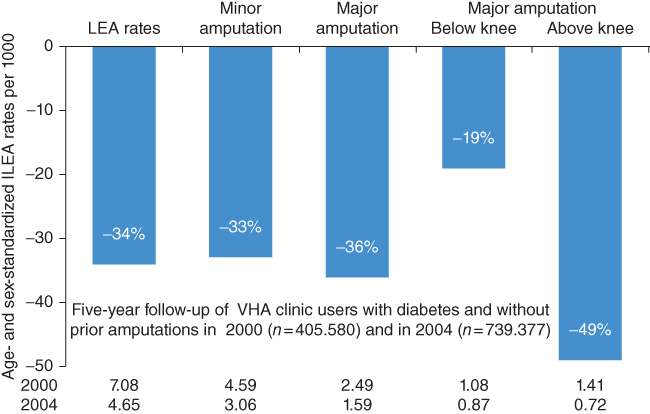
Figure 11.3 Decrease of various amputations from 2000 to 2004 among Veterans’ Health Administration health care system users. (Source: Tseng et al. 2011 [13].)
Patients with Type 2 Diabetes and Peripheral Arterial Disease in the PROactive Study
The PROactive study [15] is a double blind interventional study evaluating the effect of pioglitazone versus placebo as add-on therapy to many other cardiovascular preventive drugs. In total 5,238 patients with type 2 diabetes and a history of macrovascular disease were included and followed up for 34.5 months. At baseline, 1,274 patients had PAD and 3,964 had no previous PAD [16]. The PROactive study is a major follow-up study of patients with type 2 diabetes and PAD. Patients with PAD at baseline were older and had a longer median duration of diabetes (10 years versus 8 years in patients with no PAD at baseline). Due to the selection criteria, only 26% and 10% of patients with PAD had a previous myocardial infarction (MI) or stroke, whereas 53% and 22% of patients without PAD had a previous MI or stroke, respectively. Nevertheless, patients with diabetes and PAD at baseline (Figure 11.4) had significantly higher rates of primary and secondary composite cardiovascular disease endpoints, together with higher all-cause mortality and stroke (HR = 1.5–2.0; p < 0.0001) compared to those with no PAD. The cardiovascular disease event risk in patients with baseline PAD alone (i.e., with no other macrovascular disease) was similar to that in patients with MI alone. The amputation rate during the 2.8 years’ follow-up was 2.7% in the diabetic patients with PAD at baseline versus only 0.4% in those without PAD at baseline (HR = 6.69; p < 0.0001).
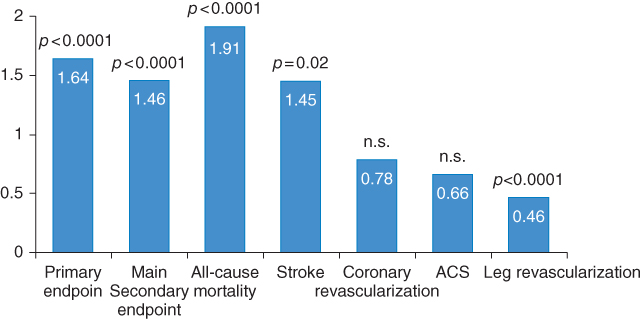
Figure 11.4 Despite strict pharmacotherapy, patients with PAD had higher rates of endpoints compared to those without. (Source: Dormandy et al. 2005 [15]. Reproduced with permission of Elsevier.)
In the PROactive study [16], pioglitazone did not alter the macrovascular event rate in patients with PAD at baseline; it is unclear why additional PAD modified the treatment effect. Leg revascularization occurred more commonly in the pioglitazone group than in the placebo group. The difference in leg revascularization occurred only in patients with PAD at baseline; there was no difference in leg revascularization between the pioglitazone and placebo group in patients who did not have PAD at entry. Moreover, the difference in leg revascularization occurred entirely in the first year.
Remarkably, a very recent secondary analysis [17] of the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial showed very promising results, indicating that the risk of new PAD is significantly decreased when insulin-sensitizing drugs (thiazolidinediones or metformin or both) are used instead of insulin-providing drugs (insulin or sulfonylurea or meglitinide). A total of 1,479 BARI 2D participants with normal ABI (0.91–1.30) were eligible for analysis. The following PAD-related outcomes were evaluated: new low ABI ≤0.9, lower-extremity revascularization, lower-extremity amputation, and a composite of the three outcomes. During an average of 4.6 years of follow-up, 303 participants experienced one or more of the outcomes listed above. Incidence of the composite outcome was significantly lower (Figure 11.5) among participants assigned to insulin-sensitizing (IS) therapy than those assigned to insulin-providing (IP) therapy (16.9% vs. 24.1%; p = 0.001). The difference was significant in time-to-event analysis (hazard ratio 0.66 [95% CI 0.51–0.83], p = 0.001) and remained significant after adjustment for in-trial HbA1c (HR 0.76 [95% CI 0.59–0.96], p = 0.02).
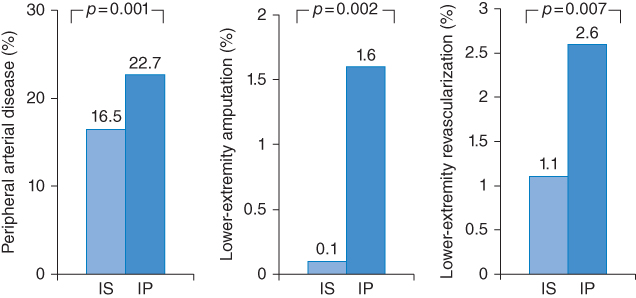
Figure 11.5 These findings clearly indicate the superiority of insulin-sensitizing (IS) versus an insulin-providing (IP) medical therapy for patients with diabetes mellitus and peripheral arterial disease. (Source: Data from Althouse 2013 [17].)
As discussed earlier, diabetic patients presenting with PAD have the highest risk for cardiovascular mortality/morbidity. A very new analysis of the association between rosiglitazone use and cardiovascular events among 2,368 patients with diabetes mellitus and coronary artery disease (CAD) in BARI 2D [18] showed totally unexpected results, which started a critical reevaluation of this drug by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Total mortality, composite death, myocardial infarction, and stroke, the individual incidence of death, myocardial infarction, stroke, congestive heart failure, and fractures, were compared during 4.5 years of follow-up among patients treated with rosiglitazone versus patients not receiving a thiazolidinedione (TZD) by use of Cox proportional hazards and Kaplan–Meier analyses that included propensity matching. After multivariable adjustment among patients treated with rosiglitazone, mortality was similar (HR 0.83; 95% CI 0.58–1.18), whereas a lower incidence of composite death, myocardial infarction, and stroke (HR 0.72; 95% CI 0.55–0.93) and stroke (HR 0.36; 95% CI 0.16–0.86) and a higher incidence of fractures (HR 1.62; 95% CI 1.05–2.51) was observed. The incidence of myocardial infarction (HR 0.77; 95% CI 0.54–1.10) and congestive heart failure (HR 1.22; 95% CI 0.84–1.82) did not differ significantly. Among propensity-matched patients, rates of major ischemic cardiovascular events and congestive heart failure were not significantly different. The authors concluded that among patients with T2DM and CAD in the BARI 2D trial, neither on-treatment nor propensity-matched analysis supported an association of rosiglitazone treatment with an increase in major ischemic cardiovascular events.
Decrease in Mortality in Patients with Peripheral Arterial Disease
Blinc et al. [19] analyzed the survival of 811 patients with PAD (defined by an ABI <0.9) in comparison with 778 control subjects in a two-year follow-up study. Mean age was 65 (SD 9) years at inclusion, with a male/female ratio of 3/2. Diabetes was present in 34% of the PAD patients, but in only 18% of the controls. All patients were treated according to the European guidelines on cardiovascular disease prevention and evaluated yearly for occurrence of death, nonfatal acute coronary syndrome, stroke, or critical limb ischemia (major events) and revascularization procedures (minor events). At baseline, classical risk factors were significantly more prevalent in the PAD group and protective cardiovascular medication was prescribed to patients with PAD more frequently than to control subjects. The overall two-year mortality was only 3.2% in the PAD group and 1.8% in the control group (p = 0.059), which is substantially lower than described in a review including older studies of patients with PAD [20]. The groups differed in two-year major event-free survival: 93.5% in PAD vs. 97.1% in controls (p < 0.017), as well as in event-free survival: 79.9% in PAD vs. 96.4% in controls (p < 0.001). Thus, patients with PAD had a borderline higher risk of all-cause death and a significantly higher risk of major and minor nonfatal cardiovascular events compared to control subjects. However, treatment according to the European guidelines on cardiovascular disease prevention resulted in encouragingly low absolute mortality and morbidity. Unfortunately, the authors did not report data for the diabetic subgroup.
Diagnosis of Peripheral Arterial Disease in Patients with Diabetes
The clinical stage of symptomatic PAD can be classified using the Fontaine staging system [21]. Fontaine stage I represents those who have PAD but are asymptomatic; stages IIa and IIb include patients with mild and moderate-to-severe intermittent claudication, respectively; those with ischemic rest pain are classified in Fontaine stage III; and patients with distal ulceration and gangrene represent Fontaine stage IV.
Diagnosing PAD in patients with diabetes is of clinical importance for two reasons. The first is to identify a patient who has a high risk of subsequent MI or stroke regardless of whether symptoms of PAD are present. Indeed, patients with diabetes and PAD have a fivefold increased risk compared to the presence of either disease alone [22–25]. An observational study less then ten years ago demonstrated that patients with diabetes and PAD stage IV (=ulcer) have a 100% mortality within six years [26].
The second reason is to elicit and treat symptoms of PAD, which may be associated with functional disability and limb loss. PAD is responsible for the majority of amputations in patients with diabetic foot syndrome in the Western world [6, 27, 28]; in other hemispheres infections, neuropathy, and diabetes per se are still the leading cause. Overall, if PAD is present in patients with diabetic foot syndrome, the likelihood of amputation is four times elevated [23].
PAD is often more subtle in its presentation in patients with diabetes than in those without diabetes. In contrast to the focal and proximal atherosclerotic lesions of PAD found typically in other high-risk patients, in diabetic patients the lesions are more likely to be more diffuse and distal [29–31]. Importantly, PAD in individuals with diabetes is usually accompanied by peripheral neuropathy with impaired sensory feedback. Thus, a classic history of claudication symptoms may be less common. In addition, diabetic autonomous neuropathy is present as well, so that arterious-venous shunts are opened and severe ischemic legs present warm with rose skin, although their low perfusion (toe pressure < 30 mmHg) should render them pale and cold. Furthermore, Moenkeberg’s mediasclerosis – incompressibility of the distal lower arteries – is frequently present in subjects with PAD and diabetes (up to 35%), resulting in false high ankle pressures and thus in a false high ABI. Johnansson et al. demonstrated that severe PAD is nearly doubled if ankle pressures are not measured alone but accompanied by toe pressure measurements [32].
Adler et al. [33] demonstrated the dilemma of a difficult diagnosis when they investigated 2,398 patients of the UKPDS for prevalent PAD. Of the classical trias for PAD, an ABI <0.8, missing distal pulses and claudication, all three were present in only 10 of the 61 patients with later PAD; in other words, in only 16%. Thus a careful inspection of the legs in patients with diabetes is mandatory and screening procedures should include toe pressure measurements.
Ankle-Brachial Index
The ankle-brachial index (ABI), a primary noninvasive screening test for PAD, is an objective measure and a risk-assessment tool with a level of sensitivity that suggests that the method may have greater utility than questionnaires and other noninvasive tools for evaluating PAD [34]. The American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend that resting ABI should be used to establish PAD diagnosis in patients with suspected PAD (subjects with exertional leg symptoms), with nonhealing wounds, and in those aged ≥70 years or ≥50 years with a history of smoking or diabetes [35, 36].
The ABI is essentially a ratio of Doppler-recorded systolic blood pressure (BP) in the lower and upper extremities and can be easily calculated. Systolic BP is measured using a Doppler stethoscope and BP cuffs on each arm and each ankle [37]. The ACC/AHA guidelines recommend selecting the higher of the two arm pressures (brachial) and the higher of the two ankle pressures (anterior tibial/dorsalis pedis or posterior tibial) to calculate the ABI [35, 36]. Thus, the right ABI = higher right ankle pressure/higher arm pressure; the left ABI = higher left ankle pressure/higher arm pressure. The index leg is generally defined as the leg with the lower ABI.
It is important to note that, because this technique for ABI calculation uses the higher pressure in the lower extremity instead of the lower pressure, it may potentially miss distal disease, thus underestimating the severity and prevalence of PAD. Therefore, several working groups have argued for using the lower of the two ankle pressures. Obviously, such a strategy improves sensitivity but lowers specifity. Nevertheless, in addition to its utility as a screening tool for PAD, where a normal ABI falls in the range of 0.91–1.30, a low ABI at rest (<0.90) indicates a high risk of PAD and provides significant evaluative and prognostic information on cardiovascular risk [38].
As it is a simple, rapid, inexpensive, and reliable method of screening asymptomatic patients for PAD, the ABI test is ideal for implementation in the primary care physician’s office. An ABI <0.9 is accepted as an indication of the presence of PAD, while values <0.5 and <0.3 indicate severe disease and critical ischemia respectively [31].
Values >1.4 may be associated with arterial incompressibility due to Mönckeberg’s sclerosis, which can be seen in diabetic patients as well as in patients with chronic renal insufficiency.
With any suspicion of incompressibility at the ankle level, the toe brachial index (the ratio of the systolic pressure of the toe to that of the arm) should be used.
Visualization of Peripheral Arterial Disease
As mentioned before, PAD is a clinical diagnosis, based on the patient’s history and ankle-brachial index measurement. However, visualization of the peripheral arteries is necessary for the therapeutic management of PAD. As recommended by the TASC II group [38], this visualization should not be limited to the localization of the target lesion itself; instead, the complete peripheral arterial tree, including the in- and out-flow, should be depicted. This is necessary to detect additional yet unknown lesions on one hand, and to identify distal runoff arteries suitable for bypass surgery on the other hand.
For several decades, digital subtraction angiography (DSA) served as the standard diagnostic modality for the visualization of patients with PAD, although it has certain disadvantages: it is an invasive and expensive examination with exposure of both the investigator and the patient to ionizing radiation [39]; moreover, DSA can visualize only the vessel lumen – the composition of vessel plaques and the surrounding structures cannot be evaluated [40].
Duplex Ultra Sonography
The first modality for noninvasive mapping of peripheral arteries was duplex ultrasound, first described in 1985 [41]. Duplex ultrasonography is a safe (no radiation or contrast agent) and cost-effective method to accurately determine the severity and location of stenosis and differentiating stenosis from occlusion [42]. B-mode or grayscale imaging displays a two-dimensional image of the artery wall and lumen, permitting a rough evaluation of the lesion and atheroma characteristics. Color-flow Doppler and pulsed-wave Doppler allow an estimation of stenosis severity on the basis of Doppler-derived velocity criteria [42, 43]. Duplex ultrasonography is an accurate method for determining the degree of stenosis or length of occlusion of the arteries supplying the lower extremity [42–44].
Although ultrasound is inexpensive and widely available, it is of limited value, being strongly dependent on investigator expertise. In addition, with the improvement in read-out MR and CT angiography, as well as fewer complications due to technical developments (less radiation, better contrast agents), duplex sonography is now considered a better pre-interventional screening technique. Before complex interventional procedures (and this is normally the case in a patient with diabetes and PAD or even a full-blown diabetic foot syndrome), CT or MR angiographies are now mandatory in order to safely plan a crural intervention in the catheterization laboratory or a femoral-pedal bypass graft. Furthermore, the sensitivity of stenosis detection was shown to decrease in the presence of additional stenosis in adjacent vessel segments [45, 46], but multiple stenoses are the normal finding in patients with diabetes and PAD [23].
Magnetic Resonance Angiography
The next noninvasive modality for the depiction of peripheral arteries is magnetic resonance (MR) angiography, which vastly improved with the development of rapid [47, 48], gadolinium-enhanced acquisition sequences, as well as multistation and hybrid protocols [49, 50] and surpassed duplex ultrasound sonography with regard to stenosis detection sensitivity [51].
Despite the rare occurrence of nephrogenic systemic fibrosis (NSF) in patients with renal failure after exposition to MR contrast media containing gadolinium chelates [52], contrast-enhanced MR angiography can still be performed in such patients using certain macro-cyclic gadolinium chelates, as recommended by the ESUR [53]. In addition, thanks to a recently developed MR sequence, nonenhanced MR angiography was shown to be as accurate as enhanced MR angiography [54]. However, this study consisted only of 25 patients; larger patient cohort studies need to be performed to confirm these results.
Finally, the use of MR angiography is limited by other contraindications such as certain cardiovascular devices (e.g. pacemakers) [55] present in a high percentage of diabetic patients due to cardiovascular comorbidities.
The quality of MR angiography has improved so that it has replaced diagnostic angiography in determining what type of intervention is feasible. The accuracy of MR angiography in identifying small runoff vessels meets or exceeds that of traditional catheter-based angiography [56]. With current technology, contrast-enhanced three-dimensional MR angiography has a sensitivity of approximately 90% and a specificity of approximately 97% in the detection of hemodynamically significant stenoses in any of the lower-extremity arteries as compared to digital subtraction angiography [57].
Computed Tomographic Angiography
The latest noninvasive modality for evaluation of patients with PAD is computed tomography (CT) angiography, which became useful with the clinical availability of multidetector CT scanners [58] and yielded highly accurate stenosis detection comparable to DSA [59].
Compared to MR angiography, CT angiography provides higher image resolution and faster image acquisition [60], both being very important for the artefact-free depiction of the lower-leg arteries of diabetic patients with severe PAD and restless legs syndrome, the latter being highly associated with diabetes mellitus type 2 [61]. Consequently, insufficient depiction of peripheral arteries is more common in MR angiography (Figure 11.6), particularly in the lower leg [62] and in the presence of endovascular stents [63], although it seems that the depiction of the in-stent lumen in MR angiography might improve with the use of blood-pooling contrast agents [64]. On the other hand, vessel wall calcifications that are highly associated with diabetes mellitus [65] decrease the diagnostic sensitivity of CT angiography (Figure 11.7). However, the information on the presence of calcification is highly important for surgical procedures such as bypass grafting, in particular in the area of the landing zone of the distal anastomosis and the clamping of the original vessel. One promising solution to this problem might be dual-energy CT scanning, which is currently under evaluation [66].
Figure 11.6 Although the patient described at the beginning of this chapter had already been treated with two stents in the symptomatic extremity, an MR angiography was performed, where the in-stent lumen (white arrowheads) was not assessable and seemed occluded (a). At DSA a few days later, the in-stent lumen presented with high-grade stenosis, but patent (b). The in-stent stenosis was treated with percutaneous transluminal angioplasty (PTA) (c). Due to the chronic progressive nature of PAD, the patient returned one year later, again with a reduced walking distance of 150 meters due to pain in the right calf. This time, CT angiography was performed. The curved planar reformations accurately depicted the in-stent-stenosis (d), which was confirmed at DSA (e) prior to PTA (f) a few days later. CFA, common femoral artery; DFA, deep femoral artery; SFA, superficial femoral artery.
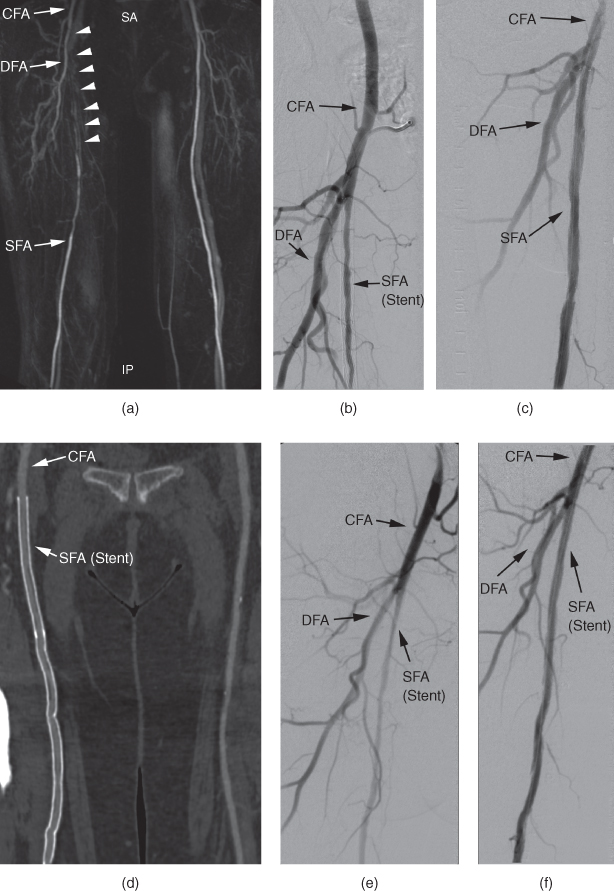
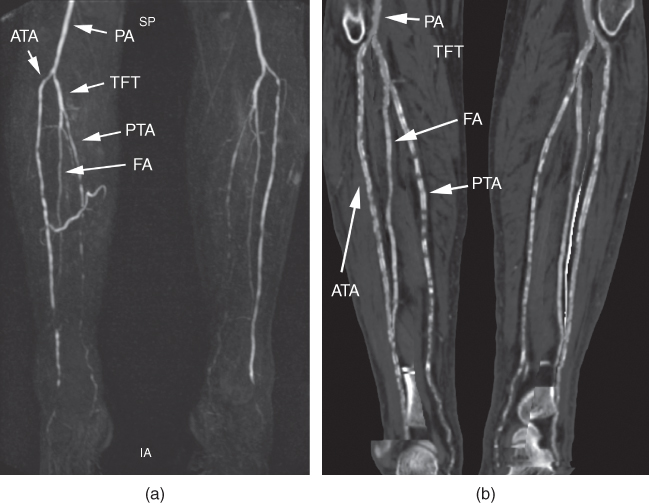
Figure 11.7 A 66-year-old male diabetic patient with chronic renal failure and hypertension presents at the outpatient department with PAD stage IV. The fourth toe on the right side was amputated in the past, and now the patient presents with ulceration at the amputation margin. MR angiography (a) clearly depicts the vessels on the lower leg, whereas the reformations of the CT angiography performed one month later (b) are of limited usability due to severe vessel wall calcifications. ATA, anterior tibial artery; FA, fibularartery; PA, popliteal artery; PTA, posterior tibial artery; TFT, tibiofibular trunk.
In addition, CT angiography has been shown to be more cost-effective than MR angiography [67] and DSA [68], although postprocessing for the generation of 3D reformations is mandatory [69], such as bone segmentation for maximum-intensity projections (MIP) and vessel tracking for multipath curved planar reformations (mpCPR) [70]. The main disadvantage of CT angiography is the use of ionizing radiation and potentially nephrotoxic contrast agents, the latter being particularly important in diabetic patients with nephropathy. At the moment, there are several efforts to reduce the required dose of radiation and contrast agents while maintaining diagnostic image quality by using automatic dose modulation and iterative reconstruction algorithms [71]. For the time being, CT angiography may be considered the gold standard for planning of peripheral crural interventions or bypass grafts [72].
Treatment Strategies in Diabetic Patients with Peripheral Arterial Disease
The aims in the management of the diabetic patient with PAD is to improve symptoms and to prevent cardiovascular morbidity and mortality. Treatment of PAD consists of three stages: lifestyle and risk factor modifications, drug therapy, and vascular interventions.
Lifestyle Modifications
Lifestyle modifications are the first step to improve metabolic and lipid abnormalities. Smoking cessation is very important, since cigarette smoking is the single most important risk factor for the development of atherosclerosis. Physical exercise improves exercise tolerance and most of the studies have shown at least a doubling in walking distance [73]. It is noteworthy that these changes were found without significant improvement in blood flow, but exercise increases cardiovascular fitness, oxidative enzyme activities, nitric oxide production, and insulin sensitivity, enhances utilization of fatty acids in calf muscles, and improves walking biomechanics as well as blood rheology. Exercise training leads to modest reductions in blood pressure, cholesterol, and glucose levels.
Rationale for Aggressive Treatment of cardiovascular Risk Factors in Peripheral Arterial Disease: Many Patients have Atherosclerosis in Other Vascular Beds
The majority of patients with early PAD are either asymptomatic or have atypical leg symptoms, with “classical” claudication in only 10–35%, therefore detection is elusive unless actively sought. Given shared risk factors, it is axiomatic that there exists a high coprevalence of atherosclerosis in other vascular beds, including the coronary arteries in PAD patients [74]. The prevalence of CAD in PAD patients ranged from 14% to 90%, which clearly reflects differences in sensitivity of the detection technique for CAD. CAD was present in 19% to 47% of PAD patients in studies using clinical history plus ECG; in 62% to 63% using stress tests (modified stress ECG or dipyridamole-stress thallium); and in 90% of subjects when coronary angiography was used [74]. Similarly, the prevalence of cerebrovascular disease (CVD) in PAD is a direct function of the sensitivity of CVD assessment. Thus, comorbid carotid stenosis >30% was reported in 51% to 72% of subjects with PAD and stenosis >70% in 25% of patients with PAD [74].
In the more advanced stage of PAD, patients may experience a multitude of problems, such as claudication, ischemic rest pain, ischemic ulcerations, repeated hospitalizations, revascularizations, and limb loss. This may lead to a poor quality of life and a high rate of depression. From the standpoint of the limb, the prognosis of patients with PAD is favorable in that the claudication remains stable in 70–80% of patients over a 10-year period. However, the rate of myocardial infarction, stroke, and cardiovascular death in patients with both symptomatic and asymptomatic PAD is markedly increased.
Recommendations for Secondary Prevention and CV Risk Reduction in PAD
As outlined earlier, patients with PAD are at a high risk of cardiovascular events and therefore benefit from aggressive secondary prevention. Current guidelines for secondary prevention and risk-reduction therapy in patients with type 2 diabetes and PAD (Table 11.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


