Chapter 6
Hypertension and Cardiovascular Disease and Its Management
Introduction
The world’s health authorities are warning about the global epidemic of diabetes mellitus (DM) [1, 2]. The increasing number of people at risk of glycemic abnormalities is directly related to the obesity pandemic in both developed and developing countries [3–5]. Millions of these people are not aware that they have or may have type 2 DM. The number of individuals with prediabetes is also quite alarming, corresponding to more than a third of adults older than 20; this is a condition that raises the risk of rather morbid complications, including microvascular and macrovascular disease (in particular kidney disease), retinopathy, blindness, amputation, and cardiovascular disease (CVD) [6–10]. The majority of people with type 2 DM are hypertensives, which gives them a two to four times greater probability of developing ischemic cardiomyopathy, kidney failure, cerebrovascular disease, and/or peripheral disease compared to patients without diabetes [11]. Hypertension may either precede or appear following type 2 DM; furthermore, the etiological factors linking diabetes and hypertension are not fully clear. Genetic factors, insulin resistance (IR), inflammation, the renin-angiotensin-aldosterone system (RAAS), sodium retention, and hyperglycemia are implicated [12–14]. The activation of the RAAS system and IR may trigger the production of reactive oxygen species and increased oxidative stress, which may lead to endothelial dysfunction and atherogenesis [12]. The macrovascular and microvascular complications are more common in type 2 diabetics with preexisting hypertension, with the RAAS emerging as a likely unifying mechanism.
Epidemiological studies have clearly shown a direct relationship between the levels of blood pressure (BP), glycemia and lipids, and the complications of diabetes [15–17]. Although “lower should be better,” the results of recent clinical trials examining the benefits of normalizing risk-factor levels have been counter-intuitive and, sometimes, disconcerting, and have called into question this belief [18, 19]. This review focuses on patients with type 2 diabetes, arterial hypertension, its association with target organ damage, and its management; it aims to provide a clear interpretation of recent trials and guidelines to help clinicians set targets for CV risk factors in individual patients.
Interaction between Type 2 Diabetes and Hypertension
The closing years of the twentieth century were marked by a series of trials highlighting the importance, and potentially large benefits, of effective hypertension treatment in patients with type 2 DM. Reduction of BP in patients with hypertension and type 2 DM is known to reduce the risk of CV events. Importantly, baseline age, percentage of patients with previous CVD, and mean BP varied substantially across the studies. This important variation in baseline characteristics could explain some of the observed differences in long-term outcomes. Additionally, new risk-lowering treatments and therapy for vascular disease have emerged over that period, such as aspirin, statins, and RAAS inhibitors; these therapeutic developments, along with temporal changes in the disease such as the increasing prevalence of obesity have well-established effects on outcomes [20, 21].
Early Trials Including Hypertensive Diabetics
The UK Prospective Diabetes Study (UKPDS) was first regarded as a cornerstone trial, with several reports comparing the effects of tight BP control on macrovascular and microvascular diabetic complications in patients with recently diagnosed type 2 diabetes [22]. A total of 758 patients were randomized to a tight control group with a BP target of <150/85 mmHg to be achieved using either captopril (400 patients) or atenolol (358 patients), with other agents added if required. A further 390 patients were allocated to less tight control (target <180/105 mmHg) using treatments other than beta-blockers and angiotensin-converting-enzyme (ACE) inhibitors. After a median follow-up of 8.4 years, the achieved BP levels in both groups differed by less than their targets, being 144/82 mmHg and 154/87 mmHg in the tight and less tight control groups, respectively. However, the differences in outcome were striking, with a reduction of 32% in the risk of death related to diabetes in the tight control group, accompanied by reductions of 44% in stroke and 34% in all macrovascular diseases. By six years of follow-up, the risk of microalbuminuria (urinary albumin ≥50 mg/L) was reduced by 29%, and fewer patients showed deterioration in retinopathy in the tight control group. The study clearly showed the benefits of BP control in preventing vascular diabetic complications when using ACE inhibitors, and the authors concluded that management of BP should have a high priority in the treatment of type 2 diabetes. Interestingly, 29% of patients in the tight control group required three or more antihypertensive treatments to achieve the BP target. A subsequent analysis revealed no significant differences in any clinical endpoint between the captopril- and atenolol-based groups [23].
Soon after the UKPDS came the Captopril Prevention Project (CAPPP), in which 10,985 patients were randomized to receive either the ACE inhibitor captopril or conventional treatment with diuretics and beta-blockers. During 6.1 years of follow-up, captopril and conventional treatment did not differ in preventing CV morbidity and mortality [24]. However, in the relatively small subgroup of 572 patients with diabetes at baseline (4.9% of the overall patient sample), the primary composite endpoint of myocardial infarction, stroke, and CV death was substantially lower in the captopril group (relative risk 0.59), and total mortality was also significantly reduced (relative risk 0.54). In this trial, the differences in outcome could not be explained by differences in BP reductions; if anything, the achieved BP levels were slightly lower with conventional treatment than with captopril in diabetic patients [25]. What these studies had in common was a clear demonstration of the very considerable benefits in terms of CV morbidity and mortality that could be achieved by antihypertensive therapies such as ACE inhibitors in patients with diabetes. However, they also gave an early indication of the controversies to come relating to specific benefits of different classes of antihypertensive drug and their combinations, and of the difficulties of clinical trial design when many effective treatments are available and optimum treatment for many patients will involve combinations of two or more drugs.
January 2000 saw the publication of the hugely influential Heart Outcomes Prevention Evaluation (HOPE) study [26]. A total of 9,297 high-risk patients with a history of vascular disease or diabetes plus one other CV risk factor were randomized to receive the ACE inhibitor ramipril or placebo for approximately 4.5 years. Study drugs were given on top of usual CV medications, except for RAAS inhibitors, which were not allowed unless required by patients’ clinical condition during the study. Ramipril reduced the incidence of the primary outcome – the composite of myocardial infarction, stroke, and CV death – by 22%, CV death by 26%, and all-cause death by 16%. An important finding was that the reduction in BP with ramipril, relative to placebo, was small (approximately 3/2 mmHg), which the authors argued was too small to account for the observed benefits. A further result was that the incidence of new-onset diabetes during the study was markedly lower in the ramipril group, with a relative risk of 0.66. There soon followed a subgroup analysis in the 3,577 patients with diabetes at baseline [27]. The BP reduction with ramipril was even smaller in this subgroup (2.4/1.0 mmHg), but the risk reductions tended to be slightly larger than in the full study population, with reductions in the primary outcome of 25%, CV death by 37%, and all-cause death by 24%. There was also a reduction in the incidence of overt nephropathy of 24%. A further analysis in patients with mild renal insufficiency [28] showed that such patients were at markedly increased risk of CV and all-cause mortality, and the relative risk reductions with ramipril were larger in patients with renal insufficiency (41% for both) than in those without (22% for CV and 10% for all-cause death).
This somewhat influential trial was soon followed by PROGRESS [29], which was primarily a study in the secondary prevention of stroke, but which had important implications for subsequent trial design, especially regarding combination therapies. Patients (n = 6,105) with history of stroke or transient ischemic attack were randomized to active treatment with perindopril, with or without the addition of the diuretic indapamide, or placebo, and mean follow-up was 3.9 years. Overall, active treatment produced a reduction of 28% in stroke and 26% in major vascular events; the benefits were similar in hypertensive and nonhypertensive patients. Approximately 42% of patients were treated with perindopril alone and 58% with the perindopril plus indapamide combination. BP was reduced by 5/3 mmHg by perindopril alone, and by 12/5 mmHg by the combination. Results in patients receiving the perindopril plus indapamide combination were dramatic, with risk reductions of 43% in stroke and 40% in major vascular events. Subsequent analysis in the 761 patients with diabetes at baseline indicated a nonsignificantly larger treatment effect in diabetic compared with nondiabetic patients, with risk reductions for stroke of 38% and 28%, respectively [30], and diabetic patients who received perindopril plus indapamide showed a dramatic 46% reduction in stroke risk.
Intensive versus Less-Intensive BP Goals
The Hypertension Optimal Treatment (HOT) study [31] and the above-mentioned UKPDS [22] were the first to assign patients randomly to less-intensive or more-intensive BP goals. The HOT study investigators randomly assigned patients to three different diastolic BP target groups (≤90 mmHg, ≤85 mmHg, and ≤80 mmHg). In the cohort of HOT participants with diabetes (n = 1,501), those in the ≤80 mmHg target group had a 51% reduced risk of CV events and 70% reduced risk of CV-related mortality compared with those in the ≤90 mmHg group after four years of follow-up. Notably, patients randomly assigned to the ≤80 mmHg target group achieved a mean BP of 144/81 mmHg, whereas those in the ≤90 mmHg target group achieved a mean BP of 148/85 mmHg.
Investigators of the Appropriate Blood Pressure Control in Diabetes (ABCD) trial [32] randomly assigned patients with diabetes and high BP [33] (mean baseline diastolic BP ≥90 mmHg) or who were normotensive [34] (mean baseline diastolic BP 80–89 mmHg) to more-intense and less-intense diastolic BP goals. Patients with hypertension enrolled in the ABCD trial were randomly assigned to a diastolic BP goal of 75 mmHg (intense control) or 80–89 mmHg (less-intense control), whereas normotensive patients were randomly assigned to a 10 mmHg decrease in diastolic BP (intense control) or no intended change in diastolic BP (less-intense control). At five years, a significant 49% decrease in risk of all-cause mortality was observed in the population with hypertension in favour of intense BP control, although this risk was a secondary outcome. No difference was observed in the primary outcome, a change in 24-hour creatinine clearance. In the normotensive population, a significant 70% reduction in relative risk of stroke was seen, although this risk was also a secondary outcome, and only 17 strokes occurred during the five years of follow-up.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial [35, 36] investigators randomly assigned patients with hypertension and diabetes (n = 4,733) to antihypertensive therapy considered intensive (targeting a systolic BP of <120 mmHg) or standard (targeting a systolic BP of <140 mmHg). The risk of nonfatal MI, nonfatal stroke, or death from causes over a mean follow-up of 4.7 years was evaluated. ACCORD is the first large randomized trial that provides the opportunity to assess the effects of lower achieved systolic BP (<120 mmHg) in patients with diabetes. Patients randomly assigned to the intensive therapy group achieved a mean BP of 119/64 mmHg, whereas those in the standard therapy group achieved a mean BP of 134/71 mmHg. The therapeutical needing’s to attain BP control in the two groups are depicted in Figure 6.2. No significant difference in risk of nonfatal MI or MI-related death was observed when comparing the intensive therapy and standard therapy groups. However, a significant 42% reduced risk of total stroke and 38% reduced risk of nonfatal stroke were seen, although the overall annual stroke rate was very low (0.32% and 0.53% in the intensive therapy and standard therapy groups, respectively). A significantly increased incidence of serious adverse events was also seen in the intensive therapy group, including hypotension, bradycardia, and arrhythmia, all of which are known to be associated with poor outcomes. The ACCORD investigators concluded that their results provide no evidence that intensive BP control reduces the rate of a composite of major events.
Figure 6.2 Main classes of antihypertensive drugs prescribed at the last study visit in patients in the intensive and standard treatment groups in the ACCORD trial. Alpha blockers, reserpine, and other antihypertensives were also prescribed in <25% of patients in either group. ACEI: angiotensin-converting-enzyme inhibitor; ARB: angiotensin-II receptor blocker; BB: beta-blocker; CCB: calcium channel blocker. (Source: Data from ACCORD Study Group (2010) [36].)
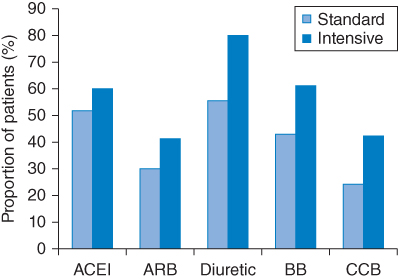
Although it may be surprising that significant reductions in the rate of events were not observed in ACCORD, it is important to note that patients in ACCORD had lower systolic BP at baseline than that achieved in the intense control groups of either HOT [31] or UKPDS [22]. This factor suggests that the benefit observed in the intense control groups of HOT and UKPDS was likely to be based on reducing systolic BP from a mean ≥160 mmHg at baseline to 144 mmHg, and that the benefit of reducing systolic BP from an average baseline value of 139 mmHg to 119 mmHg, as was observed in ACCORD, is smaller. The normotensive population in ABCD [32] is, among the patient groups studied in these trials, the one most similar to the ACCORD population, in factors such as BP, age, and percentage of patients with CVD at baseline. In both of these studies, a small, though significant, reduction in the risk of stroke was observed in the intense therapy groups.
Clinical Interpretation of Trials
The Irbesartan Diabetic Nephropathy Trial (IDNT) [37] offers the chance to assess the effects of achieved BP on outcomes in a diabetic hypertensive population with nephropathy who were followed for three years. Progressively lower achieved systolic BP to 120 mmHg predicted a decrease in related mortality and congestive heart failure, but not of MI. When patients were categorized according to achieved systolic BP of ≤120 mmHg (n = 53) or systolic BP >120 mmHg (n = 1,537), a significant increase in relative risk of all-cause mortality as well as related mortality was seen in the small group that achieved systolic BP of ≤120 mmHg. Achieving diastolic BP <85 mmHg was associated with a trend to increased all-cause mortality, a significant increase in risk of MI, but a decrease in risk of stroke. Although only 29% of IDNT participants had CVD at baseline, when categorized according to achieved systolic BP, a higher fraction of patients in the ≤120 mmHg group had a history of CVD or congestive heart failure at baseline compared with those in the >120 mmHg group [38].
The International Verapamil SR/Trandolapril (INVEST) study [39] also offers the opportunity to assess the influence of achieved systolic BP on CV outcomes in a unique population of patients with hypertension and diabetes (n = 6,400), all of whom had documented coronary artery disease at baseline and were followed for three years. INVEST participants were categorized according to achievement of a systolic BP <130 mmHg (tight control) or 130 mmHg to <140 mmHg (usual control). No difference was seen when comparing the tight control and usual control groups with regard to the rate of the primary outcome (first occurrence of all-cause death, nonfatal MI, or nonfatal stroke), nor was there any difference in the rates of nonfatal MI or nonfatal stroke when evaluated separately. However, a significant 8% increase was seen in the relative risk of all-cause mortality in the group with tight systolic control BP (p = 0.04). Extended follow-up of US participants in INVEST, using information from the National Death Index, revealed that over a total of 10 years patients in the tight systolic control BP group had a 15% excess risk of all-cause mortality compared with those in the usual systolic control BP group. This excess risk was concentrated among those with systolic BP <120 mmHg.
Available Data on Microvascular Endpoints
Some studies have also evaluated microvascular endpoints in patients with diabetes. In those receiving active treatment (perindopril and indapamide), ADVANCE investigators found a significant reduction in the development of microalbuminuria (P < 0.0001), and a borderline significant reduction in the rate of new or worsening nephropathy (p = 0.055) [40]. However, no difference between the active and control groups was found in the rate of new or worsening retinopathy, visual deterioration, or new or worsening neuropathy [41]. In UKPDS, patients in the tight control group had a 34% reduction in risk of deterioration of retinopathy (p = 0.0004), and a 47% reduction in risk of deterioration in visual acuity by three lines according to the Early Treatment of Diabetic Retinopathy Study vision chart (p = 0.004) [22], although these microvascular benefits were not sustained during UKPDS posttrial follow-up [42]. In those patients with normoalbuminuria or microalbuminuria enrolled in ABCD, BP lowering stabilized renal function in both groups; however, patients with overt albuminuria at baseline had a steady decline in creatinine clearance throughout the study regardless of BP reduction [33]. In ACCORD, patients in the intensive control group had a significantly lower estimated glomerular filtration rate (P < 0.001), more cases of elevated serum creatinine (P < 0.001), and more cases of estimated glomerular filtration rate <30 ml/min/1.73m2 (P < 0.001), although significantly fewer cases of macroalbuminuria (p = 0.009) [35]. In a substudy of ACCORD on retinopathy, intensive BP lowering was not associated with a reduction in the rate of progression of diabetic retinopathy [43].
Renal Outcomes in Hypertensive Diabetics
Nephropathy has long been recognized as an important complication of diabetes, and diabetes and hypertension are the most common causes of chronic kidney disease (CKD) [44, 45]. Worsening renal disease carries a steeply increasing risk of CV death [46] (Figure 6.1), and the complex interactions between CV disease, CKD, and diabetes are becoming more widely appreciated, if not fully understood [45, 47]. Blockade of the renin–angiotensin system is widely accepted as beneficial in terms of renal outcomes, and a series of meta-analyses have indicated that ACE inhibitors can prevent new-onset microalbuminuria and progression to macroalbuminuria, and reduce all-cause mortality in patients with diabetic nephropathy, and that angiotensin receptor blockers (ARB) have only renoprotective properties [48]. During the last 10 years, there has been a series of placebo-controlled, randomized trials of ARB in patient populations comprising or including patients with diabetes, with and without nephropathy. Characteristics of these trials are summarized in Table 6.1, including the total number of deaths that occurred in each study, as an indication of its likely power to detect a mortality benefit of active treatment, and the approximate mortality rate in the placebo group, as an indication of the risk status of the patient population. Full trial names are given in the footnote to Table 6.1, and the main results are summarized in Table 6.2.
Figure 6.1 Annual risk of cardiovascular (CV) death in patients with type 2 diabetes mellitus and different degrees of nephropathy in the UKPDS. Micro: microalbuminuria; Macro: macroalbuminuria; Elev creat: elevated plasma creatinine or renal replacement therapy. (Source: Adler et al. 2003 [47]. Reproduced with permission of Nature Publishing Group.)
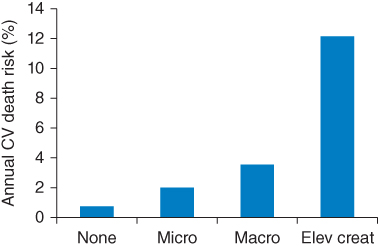
Table 6.1 Characteristics of large randomized trials with renal endpoints including patients with diabetes mellitus.
| Study | Patient characteristics | Treatments | Follow-up(years) | Baseline BP(mmHg) | BP difference vs. placebo (mmHg) | Total deaths (approximate rate)a |
| Monotherapy vs. placebo | ||||||
| IDNT (n = 1,715) [37] | Type 2 DM + nephropathy | Irbesartan (n = 579)Placebo (n = 569)Amlodipine (n = 567) | 2.6 | 159/87 | −3.3 | 263 (55) |
| RENAAL (n = 1,513) [49] | Type 2 DM + nephropathy | Losartan (n = 751)Placebo (n = 762) | 3.4 | 153/82 | −2 | 313 (60) |
| IRMA 2 (n = 590) [51] | Type 2 DM + persistent microalbuminuria | Irbesartan 150 mg (n = 195)Irbesartan 300 mg (n = 195)Placebo (n = 201) | 2.0 | 153/90 | −3 | 4 (2.5) |
| TRANSCEND (n = 5,927) [55] | Cardiovascular disease or DM with end-organ damage | Telmisartan (n = 2,954)Placebo (n = 2,972) | 4.7 | 141/82 | −4 | 713 (25) |
| DIRECT-Renal (n = 5,231) [57] | Type 1 and type 2 DM, normoalbuminuria | Candesartan (n = 2,613)Placebo (n = 2,618) | 4.7 | 118/73 | −3.3 | 99 (4) |
| Combination therapy | ||||||
| ONTARGET (n = 25,620) [69] | Cardiovascular disease or DM with end-organ damage | Ramipril (n = 8,576)Telmisartan (n
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|