Chapter 3
Kidney Disease in Diabetes
Definition
Kidney disease in diabetic patients can be caused by diabetes itself or other coexisting conditions such as hypertension or vascular disease. Diabetic kidney disease (or diabetes-associated chronic kidney disease) is a clinical diagnosis and is defined by the presence of albuminuria, often with associated abnormal kidney function (an increase in creatinine or a decrease in creatinine clearance or estimated glomerular filtration rate [eGFR]), in an appropriate clinical setting. A classic case would be a person with longstanding diabetes and coexistent diabetic retinopathy and/or neuropathy. Diabetic nephropathy is a histological diagnosis, characterized by typical histopathological features including mesangial expansion, glomerular basement membrane thickening, and glomerulosclerosis with Kimmelstiel–Wilson lesions. Diabetic kidney disease is most commonly caused by diabetic nephropathy, but other kidney pathologies may be present such as nephroangiosclerosis, atheromatous embolism, atherosclerotic renal artery disease, or glomerulonephritis.
As people with diabetic kidney disease are uncommonly biopsied unless there are clinical features suggesting a different diagnosis, most patients will not have a diagnosis of diabetic nephropathy confirmed. In this chapter, we will therefore focus on diabetic kidney disease.
Natural History and Courses of Diabetic Kidney Disease
Diabetic kidney disease is a chronic complication of diabetes and affects approximately one third of all diabetic patients [1, 2]. It is the most common cause of kidney failure requiring renal replacement therapy in Western countries [3] and can occur in both type 1 and type 2 diabetes with equivalent risks [4]. The natural history and prognosis of diabetic kidney disease differ somewhat based on the type of diabetes and whether microalbuminuria is present (Figure 3.1) [5]. In people with type 1 diabetes who have microalbuminuria, if left untreated, approximately 80% will develop macroalbuminuria (also called overt nephropathy) within 6–14 years [6, 7]. Subsequently, half of these will develop end-stage kidney disease (ESKD) over 10 years if there is still a lack of specific intervention. In contrast, approximately 20–40% of people with type 2 diabetes and microalbuminuria develop macroalbuminuria without intervention, and ESKD has been reported to develop in 20% of patients with overt nephropathy within 20 years [8]. Some of these differences may relate to the older age and greater burden of comorbidity experienced by people with type 2 diabetes for a given duration of diabetes, meaning that more of them will die of cardiovascular and other complications before developing kidney disease.
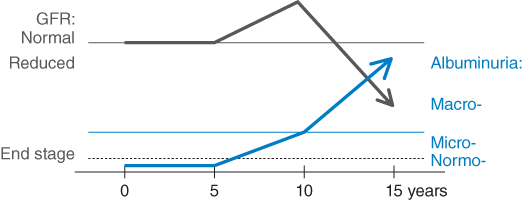
Figure 3.1 Natural history of nephropathy in diabetes. Microalbuminuria generally develops after between 5 and 15 years’ duration of diabetes. There is a high chance of progression to macro albuminuria over the next 10 years. Sometime after the onset of clinical albuminuria, the GFR begins to fall, and a large proportion of patients reach ESKD by 20 years after the onset of clinical albuminuria.
There are five stages of diabetic kidney disease: stage one has only functional changes and maintains normal glomerular structures, while stage five is ESKD (Figure 3.2). Patients with diabetic kidney disease have a markedly increased risk of cardiovascular events and mortality [9].
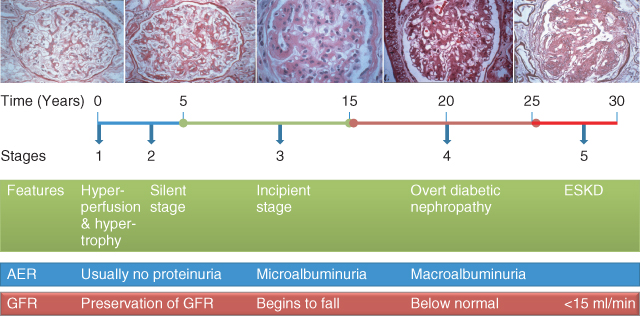
Figure 3.2 The course of diabetic nephropathy. (Source: Kidney Check Australia Taskforce, Chronic Kidney Disease and Diabetes, Workshop Module, 2013. Reproduced with permission of Kidney Health Australia.)
Pathophysiology of Diabetic Nephropathy
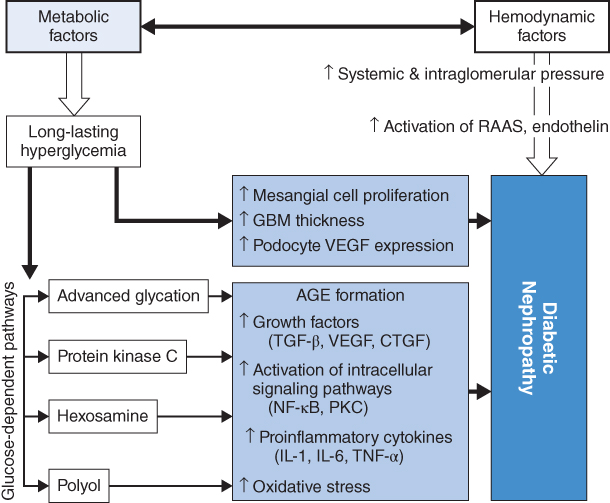
Both hemodynamic and metabolic factors play important roles in the development of diabetic nephropathy. The early signs of diabetic nephropathy are glomerular hyperperfusion due to decreased resistance of afferent and efferent arterioles of the glomerulus. This functional change further leads to an activation of the renin-angiotensin-aldosterone system and endothelin, causing structural abnormalities in the kidneys. In addition, long-standing hyperglycemia directly induces mesangial expansion, thickening of the glomerular basement membrane (GBM), and an increase in podocyte vascular endothelial growth factor (VEGF) expression. Meanwhile, an activation of advanced glycation, protein kinase C, hexosamine, and polyol also contributes to the development of diabetic nephropathy. Damage to the glomerular filtration barrier (GFB) subsequently occurs, leading to increasing urinary protein levels, inflammation, fibrosis, and eventually glomerular filtration rate (GFR) reduction and kidney failure (Figure 3.3) [10].
Figure 3.3 Metabolic factors in the pathogenesis of diabetic nephropathy. AGE: advanced glycation end product; CTGF: connective tissue growth factor; GBM: glomerular basement membrane; IL: interleukin; NF: nuclear factor; PKC: protein kinase C; RAAS: renin-angiotensin-aldosterone system; TGF: transforming growth factor; TNF: tumor necrosis factor; VEGF: vascular endothelial growth factor. (Source: Turgut 2010 [6]. Reproduced with permission of Elsevier.)
Risk Factors for Diabetic Kidney Disease
The main risk factors for the development of diabetic kidney disease include hyperglycemia, arterial hypertension, smoking, dyslipidemia, race, and genetic predisposition.
Glycemic Control
Hyperglycemia is a significant risk factor for the development of microalbuminuria in both type 1 and type 2 diabetes [11, 12]. Patients with worse glycemic control are more likely to develop diabetic kidney disease, while a reduction of HbA1c by 1% is associated with a 37% decrease in microvascular endpoints [13, 14]. More recently, data from the ADVANCE trial [2] have suggested that intensive glucose control based on a sulfonylurea reduces the risk of kidney failure by two-thirds [15].
Hypertension
Prospective studies have noted an association between the development of diabetic kidney disease and higher blood pressures [16, 17]. UKPDS analysis demonstrated that every 10 mmHg reduction in systolic blood pressure (BP) is associated with a 13% decrease in the risk of microvascular complications, with the smallest risk among those patients with systolic BP <120 mmHg [18].
Smoking
Smoking increases albuminuria and might contribute to the progression of diabetic kidney disease [19]. It is also associated with an increased risk for cardiovascular events, including a decreased survival for people with kidney failure requiring dialysis.
Dyslipidemia
Dyslipidemia, as a well-established risk factor for cardiovascular disease, is strikingly common in patients with type 2 DM, affecting almost 50% of this population [20]. Dyslipidemia is associated with the development of diabetic kidney disease in both type 1 and type 2 diabetes. In type 1 DM, increased serum triglycerides, total and LDL-cholesterol were associated with micro- and macroalbuminuria [21, 22], and high serum cholesterol also appears to contribute to GFR loss [23]. In type 2 DM, dyslipidemia is mainly attributed to insulin resistance [24] and its presence increases the risk of renal impairment [12, 16].
Genetic Predisposition
Genetic factors also play a role in diabetic kidney disease. A number of studies have shown that the angiotensin-converting enzyme (ACE) gene genotype is a potential genetic risk factor. However, definitive genetic markers have yet to be identified.
Race
The incidence of diabetic kidney disease is three- to sixfold higher in black people compared to Caucasians. Mexican Americans and Pima Indians with type 2 diabetes are also more likely to develop diabetic kidney disease.
Clinical Manifestation
Diabetic kidney disease has a heterogeneous presentation. Early stages are often asymptomatic and only detected by abnormal laboratory tests (albuminuria and changes in GFR). Albuminuria is one of the earliest detectable features of diabetic kidney disease, with a prevalence of 25% after 10 years of diabetes [25], although reduced GFR in the absence of albuminuria/proteinuria is also recognized in an increasing proportion of type 2 diabetic patients [26]. Patients with diabetic kidney disease become symptomatic once the kidney disease is severe enough, causing uremic symptoms and hypertension [26].
As diabetes manifests as a systemic disease, patients with type 1 DM almost always have other signs of diabetic microvascular complications, such as retinopathy and neuropathy. Diabetic retinopathy usually precedes the onset of overt nephropathy, while the relationship between diabetic kidney disease and retinopathy is less predictable in type 2 diabetes. Type 2 diabetics with marked proteinuria and retinopathy most likely have diabetic nephropathy, while those without retinopathy have a high frequency of nondiabetic glomerular disease. Therefore, the K/DOQI guidelines suggest that chronic kidney disease should be attributed to diabetic nephropathies in most patients with diabetes if albuminuria and diabetic retinopathy are both present [27].
Screening and Diagnosis of Diabetic Kidney Disease (Algorithm 3.1)
As diabetic kidney disease is associated with poor outcomes, early diagnosis and subsequent intervention are essential to improve prognosis. Current guidelines recommended that diabetic patients should be screened for albuminuria and GFR at least annually. Both are used as independent but additive risk factors for CKD [28–30].
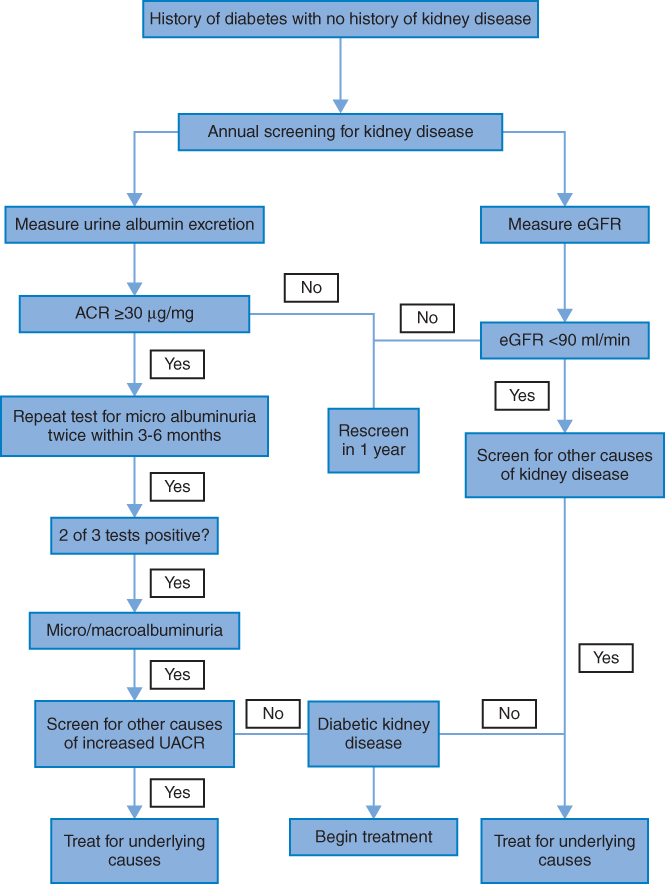
Algorithm 3.1 Screening for kidney function in people with diabetes.
Urinary Albumin Excretion (UAE)
Microalbuminuria is the earliest marker of diabetic kidney disease [31, 32] detectable with widely available laboratory tests, and the presence of microalbuminuria is associated with increased risk of cardiovascular morbidity and mortality, as well as kidney failure, in both type 1 and type 2 diabetes. Patients with proteinuria have a 2.5-fold higher mortality rate than those without proteinuria [33], and hence current guidelines suggest that albuminuria changes are an important marker of CKD progression [34, 35].
There are three ways to screen for increased urine albumin excretion (UAE): measurement of the albumin to creatinine ratio (ACR) in a random spot urine collection, 24-hour urine collection, and timed (e.g., 4-hour or overnight) urine collection [36]. UAE is most easily assessed using a spot urine ACR, preferably a first morning void spot specimen, which has been shown to correlate well with more complicated urine collections. For people with type 1 diabetes, approximately 20–30% will have microalbuminuria after a mean duration of diabetes of 15 years [37, 38]. Similarly, 25% of individuals with type 2 diabetes have microalbuminuria after 10 years, according to UKPDS [25]. Transient elevation of UAE can be seen in hyperglycemia, vigorous physical exercise, urinary tract infections, marked hypertension, heart failure, acute febrile illnesses or systemic diseases, and hematuria [39]. Therefore, abnormal albuminuria tests should be confirmed in two of three samples collected at a three- to six-month interval [40] (Table 3.1).
Table 3.1 Classification of abnormal urinary albumin excretion. [31, 41, 42]
| 24-hr urine albumin (mg/24 hr) | Overnight urine albumin (µg/24 hr) | Spot urine | Recommended follow-up | |||
| Albumin:creatinine ratio | ||||||
| Gender | mg/mmol | mg/g | ||||
| Normal | < 15 | < 10 | M | < 1.25 | < 10 | Every 1–2 years and annually for people with diabetes or hypertension |
| F | < 1.75 | < 15 | ||||
| High normal | 15 to < 30 | 10 to < 20 | M | 1.25 to < 2.5 | 10 to < 20 | |
| F | 1.75 to < 3.5 | 15 to < 30 | ||||
| Microalbuminuria | 30 to < 300 | 20 to < 200 | M | 2.5 to < 25 | 20 to < 200 | Repeat 2 times over 3–6 monthsConfirm microalbuminuria if 2 out of 3 tests are positive |
| F | 3.5 to < 35 | 30 to < 300 | ||||
| Macroalbuminuria | > 300 | > 200 | M | > 25 | > 200 | Quantify urine protein excretion by 24 hours urine protein |
Kidney function
The first manifestation of diabetic kidney disease is an increase in GFR due to hyperfiltration; however, this is difficult to detect using eGFR estimated from serum creatinine levels. People with diabetic kidney disease often develop reduced kidney function and, in fact, it can occur in the absence of albuminuria. Therefore, routine measurement of kidney function assessed by eGFR is also recommended as part of screening for diabetic kidney disease. The eGFR is derived from an estimating equation, taking serum creatinine, age, gender, body weight, and race into consideration. Normal GFR values for young individuals are 90 to 130 ml/min/l.73 m2, with a steady fall with increasing age, in the order of 10 ml/min/decade after the age of 50 years [43]. Current guidelines classify chronic kidney disease into five stages according to eGFR level and other markers of kidney disease, specifically albuminuria (Table 3.2 and Figure 3.4).
Table 3.2 Stages of chronic kidney disease. (Source: Levey et al. 2011 [38]. Reproduced with permission of Nature Publishing Group.)
| Stage | Description | eGFR (mL/min/1.73 m2) |
| 1 | Kidney damage with normal or ↑ eGFR plus persistent albuminuria | ≥ 90 |
| 2 | Kidney damage with mild ↓ eGFR plus persistent albuminuria | 60–89 |
| 3 | Moderate ↓ eGFR | 30–59 |
| 4 | Severe ↓ eGFR | 15–29 |
| 5 | Kidney failure | < 15 (or dialysis) |
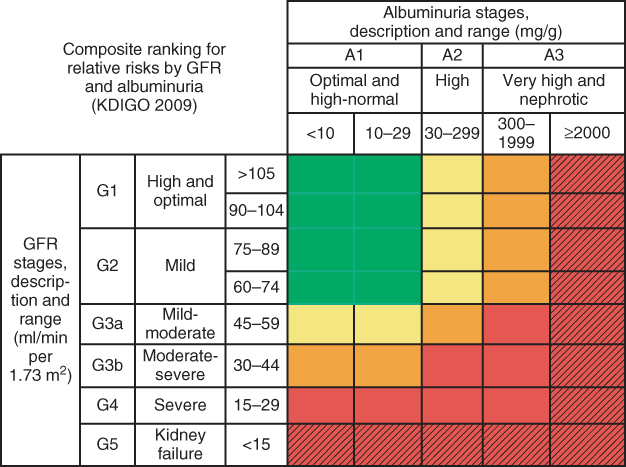
Figure 3.4 New classification of chronic kidney disease. Colors reflect the ranking of adjusted relative risk. The ranks were averaged across all five outcomes for the 28 GFR and albuminuria categories. The categories with mean rank numbers 1–8 are green, mean rank numbers 9–14 are yellow, mean rank numbers 15–21 are orange, and mean rank numbers 22–28 are red. Color for twelve additional cells with diagonal hash marks is extrapolated based on results from the meta-analysis of chronic kidney disease cohorts. The highest level of albuminuria is termed `nephrotic’ to correspond with nephrotic range albuminuria and is expressed here as > 2000 mg/g. column and row labels are combined to be consistent with the numbers of estimated GFR (eGFR) and albuminuria stages agreed on at the conference. (Source: Levey et al. 2011 [38]. Reproduced with permission of Nature Publishing Group.)
Decline of eGFR usually occurs in people with macroalbuminuria, but is less common in those with microalbuminuria. The eGFR may remain stable for years [44], but often declines with more advanced diabetic kidney disease [45].
Diabetic kidney disease is diagnosed by the presence of micro- or macro-albuminuria and/or reduced kidney function in the absence of other causes for kidney disease. Renal biopsy is usually not necessary, but may be considered in some situations to rule out important nondiabetic kidney diseases. Nondiabetic kidney disease was reported to occur in 12–38% of people with type 2 diabetes [46, 47] and should be considered in people with recent onset diabetes, acute onset of kidney disease, or clinical features suggesting another renal or systemic diagnosis.
Prognosis of Diabetic Kidney Disease
Proteinuria and abnormal kidney function are independent risk factors for renal outcomes in diabetes [28]. Diabetic kidney disease is the leading cause of ESKD requiring renal replacement therapy.
There is also an increasing recognition that diabetic kidney disease is a potent risk factor for cardiovascular disease, and is associated with an increased risk of cardiovascular morbidity and mortality [9]. Kidney disease also predicts a worse prognosis after a cardiovascular event. The US Renal Data System reported that the two-year mortality rate after a myocardial infarction (MI) was 44% among patients without CKD, compared with 58% in patients with stage 3 CKD and 68% in those with stage 4–5 CKD. Survival of patients with diabetic kidney disease is to a large extent determined by cardiovascular comorbidities.
Management of Diabetic Kidney Disease
The goal of managing diabetic kidney disease is not only to slow the progression of albuminuria and the decline of kidney function, but also to reduce the risk of cardiovascular complications. The treatment principle is a holistic approach, involving multiple and intensive strategies (Algorithm 3.2).
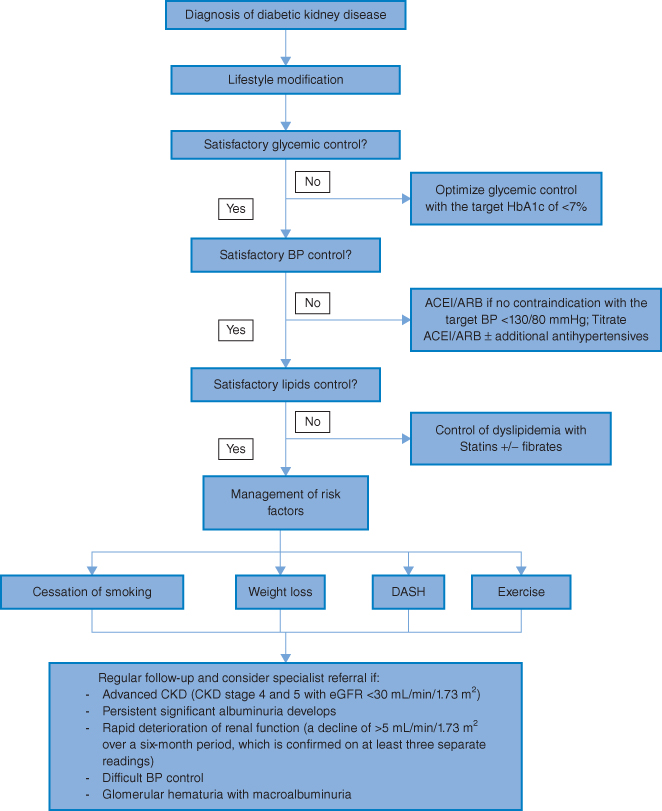
Algorithm 3.2 Management of diabetic kidney disease.
Lifestyle Modification
Weight reduction, dietary salt restriction, DASH diet (fruits, vegetables, low-fat and low-calorie diet) [48], physical activity, and moderate alcohol consumption all have been shown to reduce systolic blood pressure by 5–20 mmHg. Weight loss has also been associated with a significant reduction in microalbuminuria in obese diabetic patients [49]. These lifestyle changes should therefore be recommended to all people with diabetic kidney disease.
Glycemic Control
An accumulative body of literature has shown that intensive glycemic control reduces the risk of microalbuminuria, and slows the progression of diabetic retinopathy and neuropathy in both type 1 and type 2 DM (Table 3.3) [13, 50–52]. For example, the DCCT and UKPDS illustrated that lower HbA1c values were associated with a lower risk of developing microvascular complications, including CKD [13, 52]. Furthermore, the UKPDS follow-up study found that the risk reduction for microvascular and macrovascular complications associated with tight glycemic control in T2DM patients extended beyond the period of intensive therapy. Most recently, the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation (ADVANCE) trial [2] compared the effect of an intensive glucose-lowering strategy based on gliclazide MR (HbA1c target ≤6.5%) versus standard glucose control on renal outcome. After a median of five years, intensive glucose control reduced the risk of ESRD by 65%, microalbuminuria by 9%, macroalbuminuria by 30%, and progression of albuminuria. These results suggest that improved glucose control will prevent major kidney outcomes in people with type 2 diabetes.
Table 3.3 Clinical trials for effect of glycemic control on diabetic nephropathy.
| Name | Abbreviation | Conclusion |
| Type 1 DM | ||
| The Diabetes Control and Complications Trial | DCCT [53] | Intensive diabetes therapy can significantly reduce the risk of the development of microalbuminuria and overt nephropathy in people with diabetes. |
| The Stockholm Diabetes Intervention Study | SDIS [54] | Compared with regular treatment, intensified conventional treatment significantly reduced HbA1c level. Less retinopathy and progression of microalbuminuria was observed in ICT group, but at the expense of an increased frequency of serious hypoglycemia. |
| Type 2 DM | ||
| The United Kingdom Prospective Diabetes Study | UKPDS [51] | Intensive glucose control can significantly reduce the risk of the development of microalbuminuria and overt nephropathy in people with diabetes. |
| The Kumatomo Study | Ohkubo [55] | |



