CHAPTER 82 Thrombosis and Thromboembolism of Prosthetic Cardiac Valves and Extracardiac Prostheses
ARTIFICIAL SURFACES, COAGULATION CASCADES, THROMBOSIS, AND LYSIS
Fibrinogen is one of the major plasma proteins, often the first, that is deposited on these artificial surfaces. Once the layer of fibrinogen is absorbed onto the surface, platelets can adhere to the fibrinogen. Although surfaces vary greatly in their tendency to promote thrombosis, the reactivity of most materials to blood can be significantly increased if they are first exposed to fibrinogen.1 Other proteins also are deposited, including fibronectin (a surface protein of many cells), von Willebrand’s factor (a glycoprotein essential for the adhesion of platelets to subendothelial tissue), thrombospondin (a platelet protein secreted by activated platelets), and factor XII (Hageman’s factor, the primary activator of the intrinsic coagulation system).
Once platelets attach to the protein layer and spread out on the artificial surface, materials present in the platelet intracellular granules are secreted, including β-thromboglobulin, which inhibits prostacyclin production; platelet factor 4, which neutralizes heparin sulfate in the endothelium; and serotonin, adenosine triphosphate (ATP), and adenosine diphosphate (ADP). Synthesis of prostaglandins E and F is evident as well, suggesting that endoperoxide metabolism has taken place along with formation of thromboxane A2 from platelet arachidonic acid. Serotonin, thromboxane A2, and endoperoxide are potent vasoconstrictors and platelet stimulatory factors.2 Finally, platelet aggregation follows platelet adhesion, probably by ADP and serotonin secretion from the adherent platelets. Fibrinogen and thromboxane A2 are key in this step.
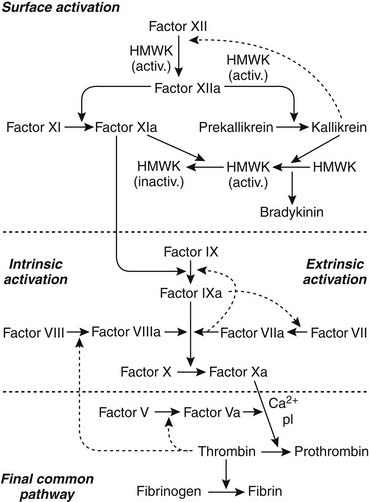
The coagulation cascade is initiated either by reaction of plasma proteins with the artificial surface to form enzymatically active components such as factor XII (intrinsic system) or by introduction of thromboplastin through exposure of subendothelial tissue to the surface (extrinsic system). Figure 82-1 is a schematic diagram of the clotting cascade. Activation of factors XIIa and XIa initiates the intrinsic system, leading to activated factor Xa. Platelets provide the phospholipid surface for this reaction. Activated factor XIIa also initiates the kininogen-kallikrein system, and kallikrein provides positive feedback for the contact activation. Kallikrein cleaves factor XII to convert it to factor XIIa, thereby accelerating contact activation. Bradykinin is also released when kallikrein cleaves high-molecular-weight kininogen. Activated high-molecular-weight kininogen can then bind more prekallikrein and factor XI to the activating surface, which further increases the reaction. In the final common pathway, prothrombin is converted to thrombin, and fibrinogen is converted to fibrin. Thrombin recruits more platelets, creating more adhesion and aggregation. A fibrin platelet clot is formed, and thrombosis occurs.
Activation of the clotting cascade on the surface of an artificial device occurs similarly whether it is on the cardiac valve, cardiopulmonary bypass system, vascular graft, extracorporeal membrane oxygenation (ECMO) circuit, mechanical assist device, or vascular catheter. It will produce thrombus formation, and macroscopic and microscopic platelet-fibrin emboli occur commonly as well. Factor XII, kallikrein, and plasmin activate the complement system and activate neutrophils, and kinin formation mediates vasodilatation, vascular permeability, and white blood cell migration. Normally, a delicate balance is maintained between these two systems so that uncontrolled clotting or hemorrhage does not occur. The coagulation cascade is initiated, and factor XII and kallikrein initiate clot lysis with conversion of plasminogen to plasmin. Antiplasmins in the circulating blood, particularly α2-antiplasmin, rapidly neutralize most of the circulating plasmin; however, plasmin also is incorporated into the clot during clot formation. The fibrin meshwork protects plasmin from antiplasmin once the plasmin is activated to plasmin, allowing fibrin degradation in the clot. In fact, many natural inhibitors offset activated procoagulant protein. Protein C, heparin, antithrombin III, protein S, thrombomodulin, prostacyclin, and plasmin all counter steps in the coagulation cascade.3
ANTICOAGULATION THERAPY
Warfarin sodium remains the most popular orally administered vitamin K antagonist used today in the United States. It blocks the formation of the four vitamin K–dependent clotting factors (prothrombin and factors VII, IX, and X), creating a buildup of their precursors. Warfarin sodium blocks the vitamin K cycle at the regeneration of reduced vitamin K, which is the active form of vitamin K (Fig. 82-2).
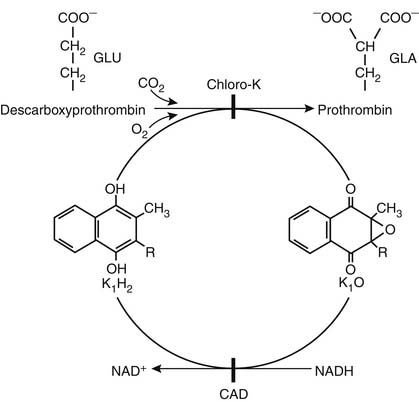
Figure 82–2 The vitamin K cycle in the formation of the vitamin K–dependent clotting factors. Vitamin K enters the body and is reduced to vitamin K1H2. K1H2 and carboxylase convert vitamin K–dependent clotting factor precursor proteins into active factors; epoxidase converts vitamin K1H2 to vitamin K1–epoxide (K1O). Reduced vitamin K1H2 is regenerated by reduced nicotinamide adenine dinucleotide (NADH) and is the warfarin-sensitive step. CAD, coumarin-type anticoagulant drugs www.lww.com.
(From O’Reilly RA. Therapeutic modalities for thrombotic disorders: vitamin K antagonists. In: Coleman RW, Hirsh J, Marder VJ, Salzman EW, editors. Hemostasis and thrombosis: basic principles and clinical practice, 2nd ed. Philadelphia: Lippincott; 1987.)
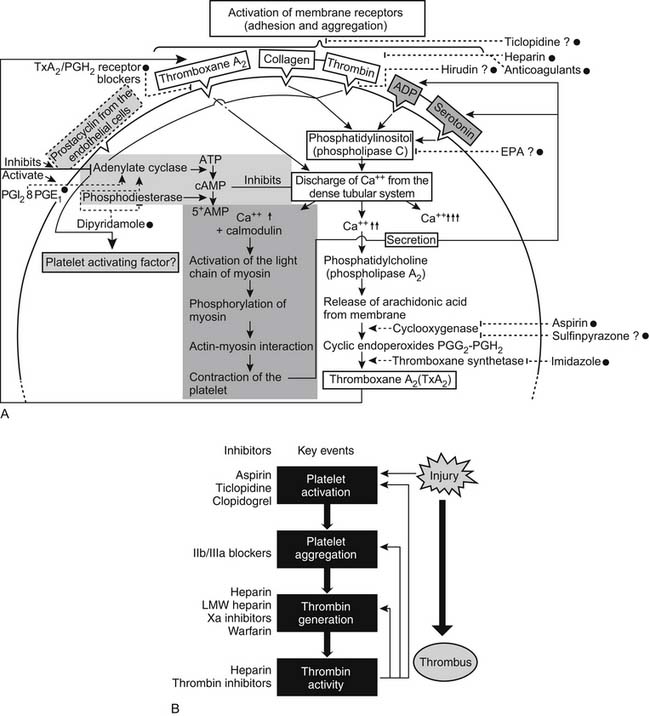
The various antiplatelet drugs have different mechanisms of action, making them more or less useful as therapeutic anticoagulation agents. Figure 82-3 is a schematic diagram of the actions of antiplatelet drugs. Aspirin inhibits platelet aggregation by irreversible acetylation of platelet cyclooxygenase, hence blocking the synthesis of prostaglandins and thromboxane A2. Aspirin prolongs the bleeding time, and its effect lasts for about 10 days (the life of the platelet). Dipyridamole, on the other hand, is a reversible platelet agent, a weak vasodilator, and a weak inhibitor of the enzyme phosphodiesterase, which degrades cyclic adenosine monophosphate (cAMP) to 5′-AMP. With this block, more cAMP is available to inhibit platelet aggregation. Sulfinpyrazone appears to reversibly block platelet prostaglandin synthesis and is another fairly weak anticoagulant. The thienopyridines ticlopidine and clopidogrel selectively inhibit ADP-induced platelet aggregation. Clopidogrel in vitro does not affect platelet aggregation. However, in vivo, clopidogrel is metabolized by the liver to several active metabolites—with these metabolites irreversibly inhibiting the P2Y12 platelet receptor. Clopidogrel supplanted ticlopidine principally because ticlopidine is associated with aplastic anemia and thrombotic thrombocytopenic purpura.4 Low-molecular-weight dextran prevents platelet adhesion and aggregation by a mechanism that also is poorly understood. At clinical dosages, neither dipyridamole nor sulfinpyrazone prolongs the bleeding time.
In clinical practice, anticoagulant therapy for artificial devices placed in the bloodstream, particularly artificial valves, is based primarily on the orally administered vitamin K antagonist warfarin sodium. Warfarin therapy is managed by following the International Normalized Ratio (INR) and maintaining it in a therapeutic range of 2 to 3.5.3,5 The INR is determined by comparing an individual clinical laboratory thromboplastin against an international reference thromboplastin. The reported International Standardized Index (ISI) is used along with the patient’s prothrombin time and the control prothrombin time to calculate the INR. This removes the significant variability between individual laboratories. The better the anticoagulation control, the better the outcome,6 but many things affect the level, including foods high in vitamin K (such as broccoli), liver disease, gut absorption, and albumin binding. Therefore, 30% to 50% of all measured values in patients with prosthetic valves may be outside the therapeutic range,5,7 and close follow-up of all patients is necessary. Studies demonstrate that the best methods for anticoagulation management involve either anticoagulation services (i.e., an anticoagulation clinic) or a self-management program.8 Indeed, self-management is associated with a much higher number of INR values in the target range than for patients managed in an anticoagulation clinic.9 This is true for both adults and children.10 Several studies with prosthetic valves11,12 have shown the benefit of adding antiplatelet therapy to warfarin.
It is well recognized that with artificial devices, particularly prosthetic heart valves, platelet survival time is decreased significantly and correlates closely with increased platelet activation and deposition.13 Addition of an antiplatelet drug (aspirin, dipyridamole, or sulfinpyrazone) normalizes platelet survival time, indicating a block in the thrombotic process.13 Several studies document a decrease in the incidence of thromboembolism in patients receiving both orally administered vitamin K antagonist anticoagulation and aspirin. However, the risk of gastrointestinal bleeding was significantly higher with the concomitant use of aspirin.5,14 Therefore, aspirin should be used with caution with warfarin because of possible excessive bleeding complications.15
Use of antiplatelet agents alone is strongly discouraged with mechanical valves, except possibly for a St. Jude valve placed in the aortic position in a child. Verrier and associates16 reported that children with mechanical aortic valves in normal sinus rhythm can be treated safely with antiplatelet agents alone with little or no risk of thrombotic events, including valve thrombosis and valve failure. Rao and colleagues17 found that aspirin plus dipyridamole was adequate for mechanical aortic valves in children, but warfarin was necessary with double valves or a valve in the mitral position. Robbins and associates18 had similar findings. Sade and colleagues19 initially indicated that the St. Jude valve could be placed in children without anticoagulation, but after 7 years of follow-up, they reported an excessive thromboembolic rate when no anticoagulation was used. Yet, Sade and colleagues were unable to answer the question of whether it was necessary to provide full anticoagulation with warfarin sodium or whether use of antiplatelet agents alone was adequate. Other studies have suggested that antiplatelet agents alone in adults and children provide adequate anticoagulation,20,21 but most patients with mechanical valves should undergo anticoagulation with warfarin unless they are at high risk for bleeding.
For tissue bioprostheses, the decision of whether to use anticoagulation is more difficult. In the initial 3- to 6-month period after implantation, the risk of thrombus formation on the sewing ring is increased, so anticoagulation is usually recommended. Most centers use warfarin sodium, but aspirin alone has been used if the patients tend to bleed.22,23 Warfarin sodium often is continued indefinitely if the patient is older or has atrial fibrillation, poor ventricular function, history of emboli, or valve placed in the mitral position. Results of a multicenter study suggest that left atrial dimension is not independently related to the development of systemic embolism in patients undergoing valve replacement.24 Edmunds3 believes that neither warfarin nor antiplatelet drugs are justified from the time of bioprosthetic valve replacement unless more than one of the mentioned risk factors for systemic embolization are present. The most recent American College of Cardiology/American Heart Association guidelines suggest that it is permissible to use warfarin for 3 months after implantation of a tissue bioprosthesis.25
Full anticoagulation with heparin is used for cardiopulmonary bypass, intra-aortic balloon assist devices, ECMO circuits, and mechanical assist devices. During bypass, activated clotting times are employed. The activated clotting time should be maintained above 400 seconds because clot has been shown to form below this value.26 If a mechanical assist device is to remain in a patient for a prolonged period, the patient may be switched to orally administered anticoagulation with warfarin sodium. A notable exception to this is the HeartMate XVE ventricular assist device system. Antiplatelet therapy alone is used with this device.27 The HeartMate II system and other axial flow ventricular assist devices at present require both antiplatelet therapy with aspirin and anticoagulation with coumadin. As experience grows with the HeartMate II, however, John and coworkers28 have described several patients who have been managed with aspirin alone because of difficulties with bleeding from coumadin. In this experience, thrombotic complications were very low. The addition of antiplatelet therapy in this group of patients, particularly those on cardiopulmonary bypass or ECMO circuits, has had significant theoretical support but no clear clinical advantage. Cardiopulmonary bypass requires that the blood come into extensive contact with the tubing, heat exchanger, reservoir, and oxygenator, either bubble or membrane. Platelets become activated, form aggregates, and release thromboxane A2. However, membrane oxygenators are much less likely to cause severe damage to the blood than are bubble oxygenators, and 20-mm Millipore filters29 are usually employed to remove the platelet aggregates that may form to prevent microemboli, particularly to the brain.
Initially, thrombosis is the major problem; but in time, hemorrhage becomes the more significant complication as heparin is continued. Platelet function is severely disturbed; thrombocytopenia occurs secondary to dilution, consumption, and sequestration of the platelets in the reticuloendothelial cell system; and fibrinolysis is initiated. For this reason, inhibition of platelet activation while the patient is on cardiopulmonary bypass has been tried with infusion of prostaglandin E1 and prostacyclin. These agents block platelet adhesion and aggregation by increasing platelet cAMP. However, hypotension from vasodilatation has been a significant problem, and several studies have shown no true benefit.30,31 New studies using aprotinin with cardiopulmonary bypass are more promising. Aprotinin, a serine protease inhibitor that has been shown to be a powerful antithrombolytic agent, is being used with increasing frequency to reduce perioperative and postoperative blood loss during open heart surgery. Aprotinin may also have some antiplatelet effect, which preserves platelet function during cardiopulmonary bypass and adds to the ability to clot postoperatively, thereby decreasing blood loss. Mohr and associates32 suggested that aprotinin has a known antifibrinolytic effect and may prevent postoperative platelet aggregation by inhibiting the high deleterious plasmin levels. This probably occurs through preservation of the glycoprotein Ib and glycoprotein IIb/IIIa receptors.
The aprotinin story in cardiac surgery merits review because the controversy surrounding this agent remains. Although no study has shown significant improved patient outcome, a meta-analysis by Levi and coworkers33 of the use of aprotinin in adult cardiac surgery demonstrated decreased surgical blood loss, decreased blood transfusion requirement, and a nearly twofold decrease in operative mortality. Equally important, aprotinin may have an important role in blocking the inflammatory response secondary to the initiation of cardiopulmonary bypass.34 On the basis of these initial reports, the Food and Drug Administration approved the use of aprotinin to reduce blood loss during coronary artery bypass surgery. However, aprotinin is quite expensive (up to $1000.00 per procedure), and concerns are still raised about increased risk of myocardial infarction, renal dysfunction, and graft occlusion.35 Indeed, two observational studies conducted by Mangano and colleagues36,37 linked aprotinin to increased risk of renal failure, stroke, myocardial infarction, and death. Two other studies also associated aprotinin use with death.38,39 Ultimately, a multicenter, randomized, blinded trial, the Blood Conservation Using Antifibrinolytics in a Randomized Trial (BART), was terminated early because of an excessive risk of death in the aprotinin group compared with the other groups, who received either aminocaproic acid or tranexamic acid.40 On the basis of this trial, Bayer Pharmaceuticals removed aprotinin from hospital pharmacies worldwide. No clear evidence exists that aprotinin is beneficial in pediatric cardiac surgery at present.41
Finally, fibrinolytic therapy has been used to declot valves to avoid high-risk emergency operations. All prosthetic valves, including bioprostheses, are subject to thrombosis.42,43 Some authors recommend that patients who are stable and less critically ill undergo surgery either to declot the valve or to remove it and replace it with another valve.44 In the case of a mechanical valve, this has usually meant replacement with a bioprosthesis. However, fibrinolytic therapy as the primary and sole method for treatment of both left- and right-sided thrombosed valves has gained considerable support.45,46
Complications of Anticoagulants
Warfarin Sodium
Bleeding is obviously the most common and most significant complication encountered with the use of warfarin, and therefore close monitoring of the prothrombin time is mandatory. Excessive amounts of warfarin that increase the prothrombin time beyond 2.5 times control and the INR above 5 will increase bleeding complications four to eight times.7 The gastrointestinal tract is the site of most bleeding complications and is often associated with preexisting disease states, such as peptic ulcer, gastritis, genitourinary lesions, cancer, and hypertension. Another significant complication of initial warfarin therapy is skin necrosis. This is secondary to a temporary hypercoagulable state induced in the capillaries when the concentration of protein C (a natural vitamin K–dependent and warfarin sodium–sensitive anticoagulant that circulates in the blood) falls before warfarin’s inhibition of factors II, IX, and X becomes effective and the desired hypocoagulable state occurs. The activated form of protein C is a powerful inactivator of factors V and VIII. Why this is limited to the skin is unknown.47 When it is used in pregnant women, warfarin can cause embryopathy in 4% to 8% of fetuses exposed in the first trimester, and exposure to warfarin in the second and third trimesters causes central nervous system abnormalities in 3% of pregnancies. Finally, prematurity, fetal hemorrhage, and stillbirth are increased in infants exposed to warfarin.48,49 For this reason, bioprostheses or allografts should preferentially be placed, when possible, in women of childbearing age.
Heparin Sulfate System
Again, the most common complication of heparin therapy is bleeding. Hemorrhagic complications occur in 10% to 20% of patients with normal hemostasis and in up to 50% of patients with thrombocytopenia or uremia.50 Thrombocytopenia has been reported in up to 31% of patients and can cause significant morbidity and mortality when it is associated with platelet clumping and thrombosis. Heparin-induced thrombocytopenia has become an increasingly recognized and important clinicopathologic syndrome. The frequency of heparin-induced thrombocytopenia among patients exposed to heparin is highly variable and related to the type of heparin, the patient population, the duration of heparin exposure, and the patient’s sex.51 The immunoglobulin G antibodies form a complex against platelet factor 4/heparin, which binds to the platelet Fc receptor, leading to platelet aggregation and release of prothrombotic microparticles. Cardiac surgery patients are prone to formation of anti–platelet factor 4/heparin antibodies. Accordingly, if the platelet count falls by more than 50% between postoperative days 5 and 14, investigation for heparin-induced thrombocytopenia antibodies should occur.51 If heparin-induced thrombocytopenia is suspected or diagnosed, a direct thrombin inhibitor (lepirudin, bivalirudin, or argatroban) should be initiated and continued until the platelet count normalizes. It is imperative that all sources of heparin be stopped if heparin-induced thrombocytopenia is suspected. This includes removal of a pulmonary artery catheter if it is heparin coated. If further anticoagulation becomes necessary, such as cardiac reoperation, one may wait for the antibodies to become undetectable and reuse heparin.52 However, this may take up to 40 days. Another alternative is use nonheparin anticoagulants such as danaparoid sodium, lepirudin, and argatroban.53 Platelet inhibition with glycoprotein IIb/IIIa antagonists followed by unfractionated heparins has also been used successfully.54
Antiplatelet Agents
Glycoprotein IIb/IIIa Agents
With the increasing use of glycoprotein IIb/IIIa agents in the catheterization laboratory, cardiac surgeons are faced with the necessity to operate emergently on some of these patients. Although the clinical safety and efficacy of agents such as abciximab (Reopro; Eli Lilly, Indianapolis, IN), tirofiban (Aggrastat; Merck & Co., Whitehouse Station, NJ), and eptifibatide (Integrilin; Schering, Kenilworth, NJ) have been shown through clinical trials of percutaneous transluminal coronary angioplasty and stent placement, there is little question that increased bleeding occurs when these patients require early (within 12 hours) cardiac surgical intervention.55 However, more recent reports suggest that urgent or emergent operations need not necessarily be delayed if glycoprotein IIb/IIIa agents are administered.56
Factor Xa Inhibitors
Fondaparinux is a pentasaccharide that binds to antithrombin in the blood. It induces a conformational change in antithrombin. This conformational change increases the affinity of antithrombin for factor Xa, potentiating the natural inhibitory effect of antithrombin for factor Xa. This agent is approved by the Food and Drug Administration for prevention and treatment of venous thromboembolism and is contraindicated in patients with a creatinine clearance of less than 30 mL/min because it is renally excreted.57
PROSTHETIC HEART VALVES
Edmunds and coworkers,58,59 and Cohn60 have detailed the need for standardized reporting of thrombotic complications: uniform definitions; stratification of the data to include the severity of the event, including death; and careful, complete long-term follow-up. Without this standardization, it is difficult to make accurate statements about results, complications, and appropriate anticoagulation therapy. Likewise, it is difficult to compare valves placed in the 1960s and 1970s and their associated complications with valves placed in the 1980s and 1990s and their complications.
Through the years, bioengineering advances have led to fewer thrombogenic materials, such as carbon Pyrolite for the St. Jude valve, and a better mechanical valve design has decreased the incidence of valve thrombosis and thromboembolism through greater central flow characteristics. However, more effective anticoagulation has been the most important factor for the decreased incidence of thrombosis in the 1980s and 1990s compared with the 1960s and 1970s, particularly with mechanical valves.6 Bioprosthetic valves have less inherent thrombogenicity, but this is due to better central flow characteristics, flexible leaflets, and sinusoidal washout more than to true thromboresistance of the preserved tissue.61 At present, two stented porcine xenograft valves are used, the Hancock and the Carpentier-Edwards glutaraldehyde-preserved valves. Several pericardial valves also have been used. These include the Ionescu-Shiley pericardial valve and the Hancock pericardial valve, both of which have been withdrawn from use in the United States. The Carpentier-Edwards pericardial valve is the only remaining pericardial valve being implanted today in the United States, although three other valves are marketed internationally. The major problem with the pericardial xenograft valves was their durability; both valves developed tearing of the cusps secondary to stress after only a few years of use. The pericardial valves used today are much better and tend to fail by calcification.46
Most prosthetic valves have a sewing ring that is covered with a Dacron or Teflon cloth. When this is exposed to the blood, an adherent layer of thrombus is laid down and serves as a blood-compatible coating. It is initially thin and delicate but later becomes invaded by well-vascularized fibrous tissue. This resists further formation of thrombus. However, if the flow pattern is abnormal, this tissue ingrowth can creep into the orifice from below the valve–sewing ring, forming an obstructive pannus. “Stentless” insertion of xenografts,62,63 including placement of porcine xenografts in the aortic root with either a cylinder of xenograft tissue or a thin Dacron support cylinder, has shown excellent early hemodynamic results without any evidence of thromboembolic events in a large number of patients. These results are encouraging, but David and colleagues recently reported excellent clinical outcomes but very poor durability of the Toronto stentless porcine valve.64 Finally, as mentioned earlier, allograft aortic valves and pulmonary valved conduits have almost normal platelet survival time and do not form thrombi.
All prosthetic valves, including bioprostheses, may develop thrombotic occlusion, but the incidence is higher for the mechanical valve, specifically the ball caged valve and the tilting disc valve. The thrombosis is usually more acute, manifesting as “sudden” valve dysfunction with clinical shock, pulmonary edema, and loss of valve click sounds in mechanical valves and muffled sounds in bioprostheses. The diagnosis is made by fluoroscopy or two-dimensional echocardiography, or both. Mortality is high without either fibrinolytic therapy or reoperation. The incidence of this complication is less than 0.3 per 100 patient-years, except for the Omniscience tilting disc valve placed in the mitral position, which has a significantly higher incidence of thrombosis.3
The incidence of all thrombotic complications, including thromboembolic events, for a mechanical aortic prosthesis is about 1% to 2% per patient-year, whereas the rate for bioprosthetic valves in the aortic position is about half that of the mechanical prostheses.3 The risk of bleeding complication is, however, significantly higher for the mechanical valves because of the use of anticoagulants, particularly warfarin sodium.
The incidence of thrombotic complications for a prosthetic mitral valve is similar in mechanical valves and bioprostheses, except for the Omniscience valve. There is little difference between the two valve positions (aortic and mitral) and between the use of a mitral prosthesis alone or a combined aortic and mitral prosthesis. However, fatal thrombotic events occur two to four times more often in patients with mechanical valves.3 Hammermeister and the Veterans Affairs Cooperative Study on Valvular Heart Disease65 concluded that after 15 years, the rates of survival for patients receiving a mechanical aortic valve replacement were higher than those for patients receiving a bioprosthetic aortic valve replacement. For patients receiving mitral valve replacement, there were no survival differences between mechanical and tissue valves. The improved survival in the mechanical aortic valve replacement group was offset by a higher rate of bleeding. Structural failure, however, was observed only with the bioprosthetic valves, and bleeding complications were statistically more frequent among patients who received mechanical valves. Hammond and associates,66 in another study of 1012 adult patients who underwent placement of either a mechanical valve or a bioprosthesis with a follow-up of 4814 patient-years, found little direct evidence to strongly support the generalized use of one type of valve over another.
Unfortunately, bioprostheses have a very high failure rate in children, probably because of accelerated calcium metabolism, and there is currently a renewed interest in the use of both fresh and commercially available frozen allograft valves for children and young adults to prevent anticoagulation. The risk of thrombotic or thromboembolic events with use of allograft aortic valves in the subcoronary position, as a free-sewn graft or by root replacement in which a cylinder including the valve is placed by the technique of Ross,12 is nearly zero without anticoagulation. Matsuki and coworkers67 reported on 555 consecutive hospital survivors who underwent isolated aortic valve replacement with a free-sewn allograft. The incidence of thromboembolism was 0.034% per patient-year, or 1 patient of the 555 studied. Results with the aortic root replacement in 108 patients described by Okita and associates68 showed no incidence of thromboembolism in 180 patient-years of follow-up. Penta and colleagues69 likewise found no incidence of thrombosis or thromboemboli in 140 consecutive patients who underwent homograft replacement of the aortic valve and were observed for a minimum of 10 years. Many of these valves were either fresh or antibiotic-preserved valves. O’Brien and associates70 reported on a comparison of aortic valve replacements with viable cryopreserved and fresh allograft valves. The freedom from thromboembolism for both groups was 97% at 10 years and 96% at 15 years; the reoperation rate for valve failure was much greater in the fresh valves. O’Brien initially believed that cryopreserved valves were superior because fibroblastic cell viability is preserved, providing durability. However, recent developments have pointed toward an immunologic basis for valve deterioration with live cells. Decellularized homografts (SynerGraft; CryoLife Inc., Kennesaw, GA) may offer better long-term results.
The fate of the aortic valve or pulmonary valve used as a conduit to reconstruct the right ventricular outflow tract is not so clear. Bull and associates71 described 249 patients who received extracardiac conduits in the right side of the heart. Of the 173 patients who survived 30 days, 72 underwent placement of xenograft conduits of various types, and 4 underwent placement of valveless tubes. The complication and reoperation rates for both valved conduit groups were similar. Calcification of the allograft tube occurred commonly, but interestingly, the obstruction tended to be at the proximal portion of the conduit where Dacron extensions, which are circumferential, were commonly placed. The development of the neointimal peel in this position led to the obstruction in more than two thirds of patients.
If a noncircumferential proximal hood is used, thrombus and neointimal peel formation is minimal, and obstruction is markedly decreased. Livi and associates72 have shown that the valve of choice for right ventricular outflow tract reconstruction is, in fact, the pulmonary allograft rather than the aortic allograft. Finally, Matsuki and associates73 have used the pulmonary autograft valve (the patient’s own pulmonary valve) to replace the aortic valve with excellent results (Ross procedure). However, this requires the reconstruction of the right ventricular outflow tract with a pulmonary or aortic allograft, essentially giving the patient disease of two valves rather than the one-valve disease the patient had originally. Despite this, Matsuki and associates73 have shown excellent results with this technique. Finally, the Contegra bovine valved jugular vein seems to be a good alternative for pediatric right ventricular outflow tract reconstruction.
Several general rules make some clinically applicable sense out of all of these sometimes conflicting data. For children, aortic valve replacement should be carried out either with a mechanical valve, such as the St. Jude bileaflet valve, or with the pulmonary autograft technique to replace the aortic valve as described by Ross in 1967.74 This has become the method of choice for aortic valve replacement in children. For mitral valve replacement in children, mechanical valves are required, and these patients should undergo full anticoagulation with warfarin. Xenograft bioprostheses should not be used because of the rapid calcification and degeneration that occurs with these valves in children. Similar guidelines should be followed for young adults. For women who are of childbearing age or wish to have children, mechanical valves should be strongly discouraged. The complications of anticoagulation, particularly with warfarin sodium and heparin, place the young woman and unborn child at too great a risk. For non-childbearing women and for men younger than 60 years, individual differences in the patients and their desired lifestyle after valve replacement should be strongly considered in choosing the valve. Finally, probably for patients older than 60 years but definitely for those older than 70 years, the use of xenograft bioprostheses (stented or stentless) or aortic allografts should be strongly considered in view of the decreased degeneration rate of these valves in older patients and the higher risk of anticoagulation in this population of patients.