Management of Malignant Pleural Effusion
Jessica S. Donington
Malignant pleural effusions (MPEs) are a common complication of advanced cancer. There are estimated to be greater than 150,000 cases of MPE each year in the United States, and approximately half of all patients with metastatic cancer will develop an MPE. Lung cancer and breast cancer account for over 75% of malignant effusions. 1 For most patients with MPE, cure is no longer an option and, for many, life expectancy is short. MPEs are clinically important because they cause significant symptoms that can severely impact the quality of life for patients with advanced cancer.
Common symptoms that result from an MPE include dyspnea, orthopnea, cough, and chest pain. Dyspnea is, by far, the most common presenting symptom and is seen in 96% of patients. 2 The mechanical impact of the pleural fluid on the diaphragm, chest wall, and compressed lung all contribute significantly to dyspnea, but may not be the only cause. Dyspnea in a patient with advanced lung cancer is very common. The majority of lung cancer patients will have dyspnea at some point during their illness and MPE is just one cause. Underlying poor lung function, parenchymal replacement by tumor, endobronchial obstruction, postobstructive pneumonia, and toxicity from treatment can also contribute to dyspnea in patients with advanced lung cancer (see Chapter 23). In a dyspneic patient with advanced lung cancer, evaluation is made for potentially correctable causes of dyspnea such as MPEs because these can be frequently palliated. It is also important to recognize the potential for multifactorial etiology for dyspnea and to note that removal of all the pleural fluid may not provide complete relief of symptoms.
Cough is another common problem in patients with advanced lung cancer, and the list of possible causes for cough is similar to that for dyspnea. Large pleural effusions are a treatable cause for cough, and lung cancer patients with cough should be evaluated for effusion and treated prior to attempting other palliative alternatives.
MALIGNANT EFFUSIONS IN NON-SMALL CELL LUNG CANCER
Malignant effusions occur in 7% to 15% of all lung cancer patients. Patients with non-small cell lung cancer (NSCLC) and associated malignant effusions are not considered to be curable, but have been classified as cT4M0, stage IIIB. These were frequently referred to as “wet IIIB” and treated in a manner very similar to stage IV disease. The International Association for the Study of Lung Cancer (IASLC) recently reviewed the current TMN staging for lung cancer and recommended several revisions (see Chapter 30). 3 One of the most significant of these revisions was to place pleural dissemination of disease, either by pleural effusion or pleural nodularity without evidence of other metastatic disease into an M1a classification, making it stage IV disease. 4 IASLC reviewed over 100,000 patients worldwide treated for primary NSCLC. Patients with pleural dissemination and without other metastatic disease (n = 488) had a median overall survival of 8 versus 13 months for other cT4M0 patients. 4 The 1- and 5-year survival patients with pleural dissemination were 36% and 2%. This was consistently worse than other T4M0 cases (where they were previously classified), but consistently better than cases with distant metastases where median survival is 4 to 7 months, hence, the new M1a classification.
PATHOGENESIS
A pleural effusion seen in association with a known lung cancer can be either malignant or paramalignant in nature. A malignant effusion is the result of direct pleural dissemination of disease. Paramalignant effusions are not a result of direct pleural involvement with tumor but are related to the primary tumor.5 Examples of paramalignant effusions include chylothorax secondary to thoracic duct obstruction, postobstructive pneumonia from an obstructing tumor with an associated
parapneumonic effusion, and an effusion secondary to hypoalbuminemia from cancer cachexia.
parapneumonic effusion, and an effusion secondary to hypoalbuminemia from cancer cachexia.
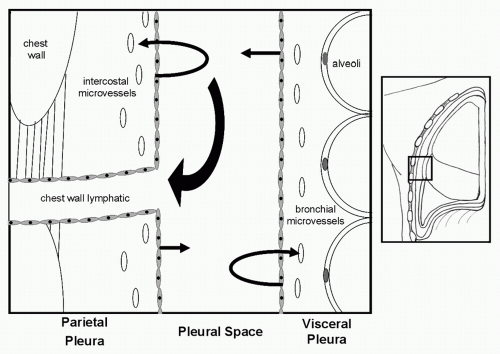
The pleural space is a moist “potential space.” Effusions occur because of an imbalance in the normal equilibrium between production and clearance of fluid in that space. Anything that results in increased fluid formation or decrease in absorption will result in an effusion (Fig. 62.1). Changes in the oncotic fluid gradient from hypoalbuminemia secondary to cancer cachexia or in the hydrostatic fluid gradient from heart failure will alter the normal forces of filtration in the intercostal and bronchial microvasculature and result in a transudative effusion. Increased capillary permeability from disease processes that directly involve the pleura itself lead exudative effusions with leakage of both proteins and fluid into the pleural space. Effusions that result from tumor implants on the pleural surface will frequently also have abundant tumor cells in the pleural fluid. Effusion caused by lung cancer, breast cancer, and mesothelioma have been noted to have elevated levels of vascular endothelial growth factor (VEGF) in the pleural tissue and fluid. VEGF is a potent inflammatory mediator as well as an important mediator of vascular permeability and angiogenesis. Elevated VEGF levels secondary to pleural tumor involvement results in an increased capillary permeability and increased fluid production from the pleural surface. 6,7
The most common mechanism for the formation of an MPE is decreased drainage of pleural fluid as a result of blockage of chest wall lymphatics.8 Blockage anywhere along the lymphatic tract, from the stomata on the surface of the parietal pleura to the mediastinal nodes can result in accumulation of pleural fluid. The development of MPEs in lung cancer are more closely related to involvement of mediastinal lymph nodes than to direct pleural involvement by disease. 9
DIAGNOSIS OF MALIGNANT PLEURAL EFFUSION
Following a careful history and physical examination, the workup for a suspected MPE proceeds through a series of diagnostic test that typically includes a chest x-ray, chest computer tomography (CT) scan, thoracentesis, pleural fluid analysis, and pleural biopsy. Many lung cancer patients will develop an effusion late in the course of their disease, but for some patients, the appearance of an effusion is their first evidence of malignancy. In patients with unilateral effusion of unknown etiology or bilateral effusions and no evidence of heart failure, diagnostic workup is recommended.
RADIOGRAPHIC IMAGING
Posterior-anterior (PA) and lateral chest x-ray can detect as little as 50 cc of fluid in the pleural space with blunting of the posterior costophrenic recess on lateral view and a pproximately 200 cc of fluid will cause blunting of the lateral recess on the PA view.10 Decubitus films can detect 100 cc of free-flowing effusion.11 Larger effusions produce a meniscus sign along the lateral chest wall and very large effusions will c ompletely
opacify (“white out”) the pleural space. Massive effusions can cause inversion of the diaphragm and a shift of the mediastinum to the contralateral side.
opacify (“white out”) the pleural space. Massive effusions can cause inversion of the diaphragm and a shift of the mediastinum to the contralateral side.
Chest CT scan with contrast has become the imaging modality of choice for better definition and visualization of a suspected MPE. CT findings characteristic of an MPE include circumferential pleural thickening, nodularity, involvement of mediastinal pleural, and evidence of a primary pulmonary tumor. Diagnostic sensitivity of these findings range from 88% to 100% and specificity from 22% to 56%.12,13 Histologic confirmation is necessary, despite a convincing image on CT scan.
Magnetic resonance imaging (MRI) provides excellent imaging of soft tissues and can provide useful information on chest wall and diaphragmatic invasion. MRI is highly sensitive for the detection of even very small pleural effusions and, with triple pulse technology, can differentiate transudative from exudative effusions, but CT scan remains the diagnostic exam of choice.
The experience with positron emission technology (PET) for the diagnosis of MPE is limited to date. In a series of 98 patients evaluated for suspected MPE, PET demonstrated sensitivity of 96.8% and specificity of 88.5% for the detection of malignancy.14 False-positive exams occurred in patients with inflammatory pleural disease, such as parapneumonic effusion. Of note, talc pleurodesis causes pleural thickening and increased activity on PET that can mimic the appearance of pleural involvement by malignancy.15
DIAGNOSTIC THORACENTESIS AND PLEURAL FLUID ANALYSIS
Patient who presents with a new pleural effusion should undergo diagnostic thoracentesis to establish transudative or exudative nature of the fluid, perform cytological evaluation, and determine the ability of the underlying lung to reexpand. Observation without thoracentesis is only recommended for patients with a well-recognized cause for the effusion, such as chronic heart failure or recent pneumonia. There are no absolute contraindications to thoracentesis; relative contraindications include small-sized effusions, bleeding disorders, anticoagulation, and mechanical ventilation. Complications associated with thoracentesis include pneumothorax, hemothorax, infection, and hemoptysis. Thoracentesis has traditionally been performed as a “blind” procedure where the needle is placed based on standard positioning and the appearance of fluid on radiographic imaging. Several studies have indicated that ultrasound guidance can assist even experienced physicians in selecting appropriate puncture site. 14,16,17,18
TABLE 62.1 Criteria for Establishment of Exudative Pleural Effusion | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The gross appearance of pleural fluid at thoracentesis can be suggestive of an MPE, if it is hemorrhagic or opalescent. Half of all hemorrhagic effusions are malignant and 11% of MPEs are bloody in nature. 19 The majority (90% to 97%) of MPEs are exudative, but malignancy is not the only cause of an exudative effusion. Inflammatory causes are also common, because anything that increases capillary permeability in the pleural space will result in leakage of both fluid and protein into the pleural space. Although, the presence of an unexplained exudative effusion is worrisome for malignancy, the absence of transudative properties does not rule out a malignant etiology. 20 Exudative properties are most commonly defined on the basis of the Light’s criteria, which is outlined in Table 62.1. Overall diagnostic accuracy of Light’s criteria is 93%. 21
Pleural fluid from thoracentesis should be evaluated for lactate dehydrogenase (LDH), total protein, pH, glucose, and cell count. LDH and total protein are components of Light’s criteria for determination of exudate versus transudate. Low glucose and pH are common in pleural space infections but are also seen in up to 30% of MPEs and can be prognostic with regard to palliation of effusion and overall survival. 22,23 The diagnostic yield from the cytological examination of fluid from thoracentesis is variable, ranging from
62% to 90%. 24,25,26,27,28,29 Increasing the volume of pleural fluid sent for cytological evaluation does not increase the sensitivity. 28 If a diagnosis is not made by initial thoracentesis, a second drainage can be attempted with approximately a 25% increase in yield, but the increase in yield drops dramatically after two attempts.30 Repeated diagnostic aspirations beyond two are not recommended because of low diagnostic yield and increasing risk for infection and loculation. Closed pleural biopsy with an Abrams or Cope needle is often attempted when initial cytology is negative. Although the diagnostic yield from closed biopsy when combined with cytology can be as high as 80%,31 it has very small diagnostic yield in cases where the initial cytological evaluation was negative.26 Therefore, if two consecutive percutaneous drainages provide no diagnosis, thoracoscopy is recommended. Video-assisted thoracoscopic surgery (VATS) allows for wide examination of the pleural space and for large visually directed pleural biopsies. Diagnostic sensitivity for VATS procedures is reported at greater than 90% with specificity of 100% and perioperative mortality is less than 0.5%. 32,33
62% to 90%. 24,25,26,27,28,29 Increasing the volume of pleural fluid sent for cytological evaluation does not increase the sensitivity. 28 If a diagnosis is not made by initial thoracentesis, a second drainage can be attempted with approximately a 25% increase in yield, but the increase in yield drops dramatically after two attempts.30 Repeated diagnostic aspirations beyond two are not recommended because of low diagnostic yield and increasing risk for infection and loculation. Closed pleural biopsy with an Abrams or Cope needle is often attempted when initial cytology is negative. Although the diagnostic yield from closed biopsy when combined with cytology can be as high as 80%,31 it has very small diagnostic yield in cases where the initial cytological evaluation was negative.26 Therefore, if two consecutive percutaneous drainages provide no diagnosis, thoracoscopy is recommended. Video-assisted thoracoscopic surgery (VATS) allows for wide examination of the pleural space and for large visually directed pleural biopsies. Diagnostic sensitivity for VATS procedures is reported at greater than 90% with specificity of 100% and perioperative mortality is less than 0.5%. 32,33
The addition of tumor marker evaluation to the analysis of pleural fluid has been investigated. Carcinoembryonic antigen (CEA), carbohydrate antigen 15-3 (CA 15-3), cytokeratin 19, and cancer antigen 125 (CA-125) analysis can all be performed, but their diagnostic value remains limited.34 No single marker has sufficient specificity to add to routine practice. In cases of NSCLC, epidermal growth factor receptor (EGFR) analysis of MPE is proving useful. EGFR mutations can be detected from cells in MPEs, helping to identify a group of patients likely to benefit from EGFR-targeted therapies.35 DNA methylation analysis appears to carry significant diagnostic value in MPEs. Brock et al.36 detected DNA methylation in 59% of MPEs but in none of the benign effusions evaluated. The addition of methylation studies to standard cytological evaluation increases both the sensitivity and the negative predictive value compared to cytology alone. 29,36
TREATMENT
The goal of treatment in a patient with a malignant effusion from lung cancer or other tumors is palliation of symptoms. MPEs occur in a diverse patient population with variable life expectancies. A small percentage of patients are robust and will have life expectancies of months to years, whereas many patients are frail with advanced disease and significant cancerrelated comorbidity with life expectancy of only days to weeks. It is important to appropriately tailor therapy to the best needs of the individual patient. Successful palliation of an MPE is judged by long-term relief of symptoms related to the effusion and no evidence of reaccumulation of fluid on chest radiograph until death. Removal of the effusion, improvement in symptoms, and prevention of reaccumulation do not signify a cure or prolong expected survival from the underlying malignancy, but inappropriate management of an MPE can shorten expected survival by compromising respiratory function.
The two most common treatment options currently used for MPE are pleurodesis or the insertion of a chronic indwelling tunneled pleural catheter. There are numerous other treatment options from noninvasive approaches such as observation or repeated thoracentesis, to the very aggressive procedures such as pleurectomy. The use of systemic chemotherapy has limited use in palliation form MPE except in small cell lung cancer, where many patients will respond to chemotherapy with resolution of their effusion and associated dyspnea. 37 Similarly, MPEs rarely respond to mediastinal radiation except in the case of some lymphomas where the effusion is a result of obstruction of mediastinal lymphatics that can be relieved following radiation. 38
Observation Observation alone is recommended for those patients who are asymptomatic from their effusion or in the small number of patients (<2%) in whom there is no fluid reaccumulation following thoracentesis.1,39 Observation and supportive care are also reasonable options for those patients who are very frail and whose life expectancy is in days. Supportive care in this situation also includes the use of opioids and oxygen therapy to ease the anxiety associated with dyspnea.40
Serial Thoracentesis Thoracentesis is very effective at acutely alleviating symptoms associated with MPE and has an important role in the diagnosis and planning treatment, but it has limited value as therapeutic approach. From 98% to 100% of MPE associated with lung cancer will reoccur within the first month of the initial thoracentesis.1,39 Serial thoracentesis usually need to be performed frequently with an interval dependent on the rate of fluid accumulation. Repeated thoracentesis can lead to pneumothorax, fluid loculation, and empyema. This approach is only recommended in patients whose life expectancy is days to weeks, those who are too frail for pleurodesis or insertion of a chronic indwelling tunneled pleural catheter, or in the very rare patient with a very slowly reaccumulating effusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree