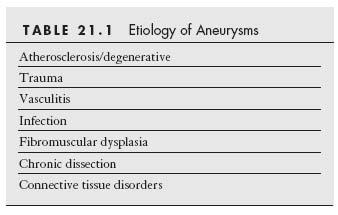
An aneurysm has been traditionally defined as a localized dilation of an arterial segment by more than 50% of the reference diameter (1). Although more than 95% of the arterial aneurysms are located in the aorta, they may develop in any artery of the body. A true aneurysm is one that involves dilation of all three layers (i.e., adventitia, media, and intima) of the arterial wall, characterized pathologically by extensive atrophy of the media. In contrast, a false aneurysm, or pseudoaneurysm, is the result of the disruption of at least one of the three layers of the vessel wall, and blood is contained within the adventitia or by the surrounding tissue. While pseudoaneurysms are mainly caused by trauma, a myriad of etiologies are related to the formation of true aneurysms (Table 21.1).
With the refinement of endovascular technologies, many aneurysms may be managed by an endovascular approach, rather than by surgery. Endovascular therapy has the advantage of lowering morbidity (e.g., perioperative myocardial infarction, infection, prolonged recovery) by obviating the need for general anesthesia, avoiding blood loss, and by reducing inhospital recuperation time, when compared to surgical treatment. This chapter reviews the contemporary endovascular management of the aneurysms in the extra-aortic vessels.
INDICATIONS FOR REPAIR
In general, the indications for aneurysm repair include (1) symptom(s) caused by the aneurysm (e.g., thromboembolism, compression on surrounding tissues and organs); (2) prevention of impending rupture; and (3) evidence of aneurysm expansion detected by serial imaging studies.
It is important to realize that angiography only provides a lumenogram of an artery, and the actual size of the aneurysm may be underestimated in the presence of a layered, intramural thrombus. Imaging modalities such as ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) should be performed in order to identify the vessel wall and to provide the actual measurement of the size of an aneurysm.
STRATEGIES OF ENDOVASCULAR TREATMENT OF ANEURYSMS
Many factors should be taken into consideration when determining the appropriate management strategy for an aneurysm. These include the patient’s clinical presentation, comorbidities, the location, size, and morphology (saccular or fusiform) of the aneurysm, and the anatomy of the vascular bed involved. The aim of the treatment is to exclude the aneurysmal sac, while maintaining the long-term patency of the parent vessel.
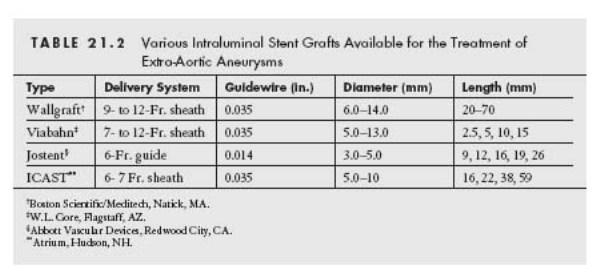
Endovascular strategies for aneurysm repair may be classified into two categories, namely stent graft placement (Table 21.2 and Figs. 21.1 and 21.2) and embolization (Table 21.3).
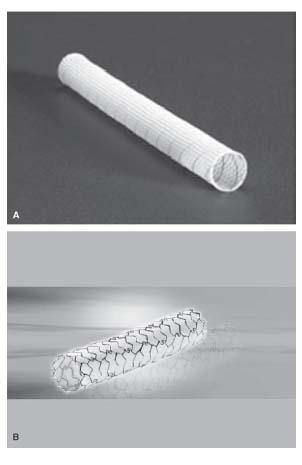
Stent grafts exclude the aneurysm and maintain long-term patency of the main vessel. Both self-expanding and balloon-expandable stent grafts are available. For a fusiform aneurysm, the presence of a proximal and distal neck is necessary for successful endovascular repair. The choice of stent graft largely depends on the location of the aneurysm, and the size of the parent vessel. For a large vessel subject to compression or flexion, a self-expanding Wallgraft (Fig. 21.2A) or Viabahn may be used, with a large delivery sheath (Table 21.2).
The Wallgraft endoprosthesis is composed of a stainless steel, monofilament wire, covered by polyethylene (PET) graft material. The Viabahn endoprosthesis is made up of polytetrafluoroethylene (PTFE) lining, with an external nitinol skeleton extending along its entire length. Since a self-expanding stent graft may resist deformation, it is ideally suited for the treatment of aneurysms located in vascular regions that are subject to compression or flexion, such as those in the common carotid artery, proximal internal carotid artery, axillary artery, femoral artery, and popliteal artery (Figs. 21.3 and 21.4). The size of a self-expanding stent graft should be 0.5 to 1.0 mm larger than the estimated reference diameter of the target vessel, in order to minimize shortening of the stent graft, and to ensure adequate apposition of the stent with the vessel wall.
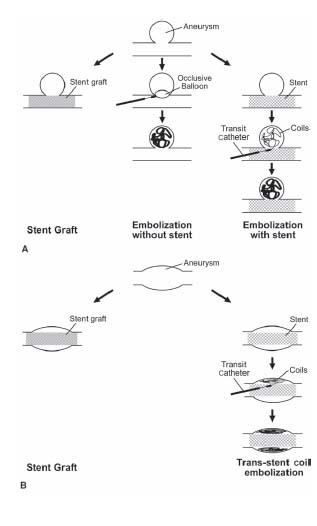
Figure 21.1 • Schematic diagrams depicting the strategies of endovascular repair of (A) a saccular aneurysm and (B) a fusiform aneurysm. In (A), the aneurysm may be isolated by a stent graft within the parent artery, treated with embolization coil(s) alone, or bare metal stent placement in the parent vessel and embolization of the aneurysm. In (B), the aneurysm may be treated with a stent graft or a bare metal stent combined with embolization coil(s) within the peri-graft space to induce thrombosis.
Figure 21.2 • A: Wallgraft™. B: Jostent™ covered stent.
For vessels with smaller diameters (up to 5.0 mm), a balloon-expandable, covered stent (e.g., Jostent) may be used with the advantage of precise stent placement, and immediate exclusion of the aneurysm (Figs. 21.2B and 21.5). The Jostent is constructed by sandwiching an ultrathin layer of PTFE graft material between two stents that are welded at the ends. Recently, pre-mounted Jostents have become available. The diameter of these stents is chosen to match the reference diameter of the target vessel. Occasionally, operators may find it useful to perform intravascular ultrasonography, to determine the reference vessel diameter. High-pressure inflation (12 to 18 atm) should be used to ensure full expansion and apposition of the stent. For aneurysms in larger vessels (i.e., >75 mm) that are not subject to compression or flexion, the iCAST covered balloon-expandable stent offers an important low-profile alternative to self-expanding covered stents.
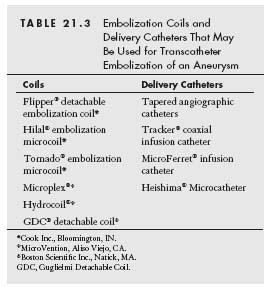
Before discussing embolization strategies for the treatment of aneurysms, it is worth providing a brief outline of the coils that have been developed for vascular occlusion. Most coils are composed of stainless steel or platinum, with attached Dacron strands to enhance thrombogenicity. The coils are delivered from within a hollow, linear, metal tube (called the loading canula/cartridge) and when released, assume a variety of shapes: straight, curved, helical, or tapered helical (e.g., tornado). Coils are also defined by the diameter of the metal coil (e.g., 0.018″ to 0.052″), the diameter assumed by the unconstrained coil (e.g., 2 to 10 mm), and the linear length of the coil when constrained in its linear form by the loading canula (e.g., 2 to 10 cm).
In general, delivery of the coil is achieved by putting the metal loading canula into the hub of the delivery catheter whose tip has been positioned at the desired location for coil delivery. The coil is then extruded from the loading canula into the delivery catheter by pushing a wire guide into the proximal end of the loading canula. Continued advancement of the wire results in extrusion of the coil from the distal end of the delivery catheter.
For each coil, the manufacturer provides the recommended minimum and maximum internal diameter of the delivery catheter. Strict adherence to these recommendations is advised. A variety of delivery catheters are available (Table 21.3). The use of polyurethane catheters is discouraged because of the risk of the coil becoming lodged in the catheter. For each coil, the manufacturer will also recommend the appropriate diameter of the wire guide. This will vary from 0.018″ to 0.035″. While certain guidewires are available for use with specific delivery catheters (e.g., Tracker), the stiff end of any appropriately sized wire may be used. Attention to coil sizing during treatment of aneurysms is important. If a coil is too small, there is an increased risk of embolization. Alternatively, using too large a coil will result in the coil remaining elongated, and decrease the likelihood of occlusion of the aneurysmal sac. In addition, the proximal end of the coil may protrude through the neck of the aneurysm, or the interstices of a stent, and provide a nidus for thrombus formation.
Saccular aneurysms may be effectively treated using coil embolization, with or without stent placement in the main vessel. When embolization coils are used, placing a stent within the parent vessel may prevent coil herniation and hence promote thrombogenesis within the aneurysm (Figs. 21.1A,B). For a narrow neck, saccular aneurysm, an occlusion balloon catheter (e.g., Fogarty balloon catheter) may be first advanced into the aneurysm over a directionally controlled guidewire (Fig. 21.1A). With the balloon inflated, the catheter is then pulled back snugly to the neck, to prevent coil embolization. Alternatively, coil embolization may be performed via a microcatheter (e.g., Transit catheter) within the aneurysm (Figs. 21.1A and 21.6).
For fusiform aneurysms, a bare metal, self-expanding stent may be deployed across the lesion (Fig. 21.1B). A Transit catheter may then be placed within the aneurysm, through the stent struts, over a 0.014″ guidewire. The guidewire is then removed, and coils may be deployed into the aneurysmal cavity by pushing with the stiff end of a 0.018″ wire through the Transit catheter. The number of coils needed is determined by the size of the cavity. Attention needs to be paid to avoid disengaging the Transit catheter out of the aneurysm while deploying the coils. The diameter of the coil should be about 1 mm larger than that of the target vessel.
Endoleaks (Table 21.4) occur as a result of failure of a graft to seal an aneurysm completely and are typically classified into four types (see Chapter 18, p 295–299 for full details).
Persistence of an endoleak may result in continuous aneurysmal expansion and rupture. Patients should be followed up with ultrasound, CT angiography, or invasive angiography, to detect the presence of any endoleak after endovascular repair of an aneurysm. The specific therapy for an endoleak will depend on the particular mechanism of the endoleak. (Fig. 21.4).
SPECIFIC CONSIDERATIONS BASED ON ANEURYSM LOCATION
Extracranial Carotid Artery
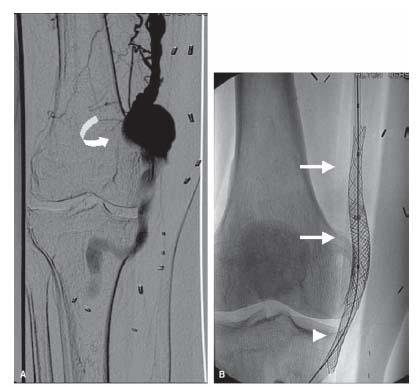
Aneurysms located in the common carotid artery are usually caused by atherosclerosis, trauma, or post-infectious changes, whereas those in the carotid bulb are most commonly seen after surgical endarterectomy. Carotid aneurysms related to fibromuscular dysplasia (FMD) are usually located in the distal internal carotid artery. The presence of carotid FMD should prompt a search for FMD in other arterial territories, particularly within the vertebral, renal, and intracranial arteries (2). Spontaneous dissections are frequently associated with FMD (Fig. 21.5).
Extracranial carotid aneurysms may be asymptomatic and may only be detectable by palpation underneath the angle of the jaw on physical examination. Occasionally, the lesions may be associated with cervical pain, transient ischemic attacks, or stroke resulting from embolization. Patients may provide a history of invasive tests or cervical manipulation (e.g., insertion of internal jugular venous catheter). Although ultrasonography, CT angiography, or magnetic resonance angiography usually is the initial imaging modality, contrast angiography is often required both for confirmation of the diagnosis and guidance of management. Knowledge of the intracranial vascular anatomy and any associated cerebrovascular obstructive disease or dissection is necessary in order to select the appropriate management strategy.
Figure 21.3 • Endovascular treatment of popliteal artery aneurysm. This patient had a history of coronary artery bypass surgery, ischemic cardiomyopathy, and infrarenal abdominal aortic aneurysm repair with an endovascular graft. Endovascular exclusion of the popliteal artery aneurysm (curved arrow) was performed because of multiple embolic events to the foot (A). Antegrade arterial access with a 12-Fr. sheath was established in the right common femoral artery. Over a stiff-angled glidewire, two 10-mm-diameter × 70-mm-long Wallgraft stents (arrows) were placed sequentially across the popliteal artery aneurysm. Post-dilation with 10-mm-diameter × 40-mm-long balloon catheter was performed. A residual leak was demonstrated on repeat angiography and this necessitated placement of a 12-mm-diameter × 50-mm-long Wallgraft (arrowhead) distally followed by post-dilation with a 12-mm-diameter × 40-mm-long balloon catheter (B). Final angiogram revealed adequate exclusion of the aneurysm (C). Aspirin, clopidogrel, and warfarin were provided after the procedure.
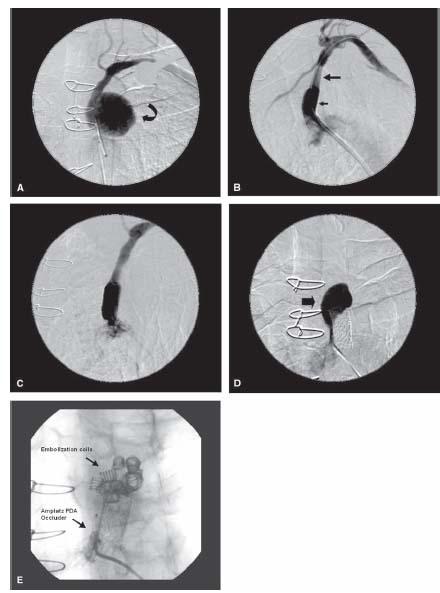
Figure 21.4 • Endovascular repair of left subclavian artery pseudoaneurysm. This 79-year-old man complained of left upper extremity numbness following coronary bypass surgery (involving the use of a left internal mammary artery conduit). He had a history of hypertension and surgical repair of an abdominal aortic aneurysm 20 years previously. A large pseudoaneurysm (curved arrow) was demonstrated in the proximal left subclavian artery (A). Via a 12-Fr. sheath inserted through the right common femoral artery, an 11-mm-diameter × 50-mm-long Viabahn endoprosthesis (large arrow) was advanced over a 0.018″ Roadrunner wire and was deployed across the aneurysm. Post-dilation was performed with a 12-mm-diameter × 40-mm-long balloon catheter. A 12-mm-diameter × 26-mm-long Intrastent® (small arrow) was deployed within the inflow of the covered stent in a bid to seal off the residual leak (B). A year later, repeat angiography revealed a patent subclavian artery and persistence of a large perigraft aneurysmal sac (C,D, block arrow). Via a 4-Fr. multipurpose catheter, a total of three Flipper® detachable embolization coils (0.035″ × 5 cm × 8 mm, two 0.035″ × 12 cm × 8 mm), two Hilal® embolization coils (0.038″ × 8 cm × 5 mm), and eight embolization coils (0.038″ × 8 cm × 5 mm) were deployed. An Amplatz PDA® occluder (8 mm × 6 mm) was deployed via a 7-Fr. Shuttle sheath in order to seal the entrance of the aneurysm (E).
The natural history of an untreated carotid aneurysm is not known. In the past, surgical repair was recommended because cerebral ischemia was common, and most patients remained asymptomatic after surgery (3–5). Endovascular repair has become a more attractive approach because it eliminates the use of general anesthesia and the potential complications related to surgery (e.g., cranial nerve palsy or stroke). Percutaneous treatment is the therapy of choice for patients with appropriate anatomic features.
The technique of carotid angiography and choice of guide catheters has been described in Chapter 10. During angiography, it is critical to identify the location of the neck of the aneurysm, in order to ensure successful treatment of the aneurysm. For aneurysms (saccular or fusiform) and pseudoaneurysms located in the common carotid artery or at the carotid bifurcation, coil embolization or stent graft placement has been performed successfully (6–9). The balloonexpandable covered stent, Jostent, has been used for treatment of distal internal carotid artery aneurysms (Fig. 21.5) (10). All patients should undergo ultrasound and Doppler study prior to discharge, and again in 1 month, 6 months, 1 year, and annually thereafter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree