Chapter 58 Management of Complications after Endovascular Abdominal Aortic Aneurysm Repair
Endovascular abdominal aortic aneurysm repair (EVAR) for abdominal aortic aneurysms (AAAs) has gained wide acceptance since it was first reported in 1991.1 Randomized controlled trials have shown decreased short-term morbidity and mortality when compared with open controls.2,3 Refinements in device design, such as the development of lower-profile delivery systems, and improvements in preoperative imaging for procedure planning and device sizing have reduced the complications associated with EVAR. However, as with any therapy, the potential for complications exists. This chapter reviews the common short- and long-term complications of EVAR, as well as their prevention and management options.
Early Complications
Access
In order for EVAR to be considered as an option for a patient, the access vessels (the femoral and iliac arteries) must be fully evaluated and deemed to be of adequate caliber and acceptable tortuosity to accommodate the device and the delivery system. Challenges related to the access vessels can result in significant perioperative morbidity and potential mortality.4,5
As device delivery profiles have decreased in size, fully percutaneous endovascular aneurysm repair (P-EVAR) has gained popularity as a technique. Most authors report using percutaneous closure for up to 24-French sheaths. A recent systematic review of all articles published from January 1991 to July 2009 on P-EVAR found an overall access related complication rate of 4.4% (95% confidence interval, 3.5 to 5.3). Increased incidence of access related complications were associated with larger sheath size and obesity.6
Major complications of the percutaneous approach are due to failure to adequately close the femoral arteriotomy. These complications include retroperitoneal hemorrhage and femoral artery pseudoaneurysm formation. In most cases, the failure is immediately obvious and open repair of the femoral artery is performed. One study examined 279 femoral arteries that were accessed percutaneously with an immediate failure rate of 6%.7 The midterm follow-up data on this group showed 3 of 156 (1.9%) having late complications: a femoral artery dissection and 2 pseudoaneurysms.8
Relative contraindications to percutaneous repair, which may be associated with a higher rate of complication, include: circumferential or anterior calcifications of the femoral arteries, small access vessel size, severe groin scarring, femoral bifurcation above the inguinal ligament, and large groin pannus.9 A high puncture site can be associated with hemorrhage on mobilization, whereas a low puncture site can be associated with vessel occlusion. Ultrasound guidance can be a useful adjunctive technique to find the optimal puncture site and avoid potential complications.
Iliac Artery Complications
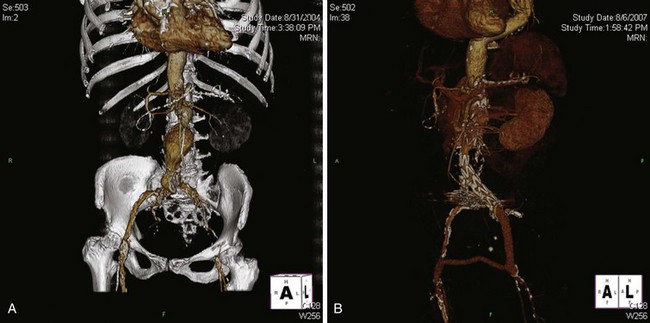
Iliac artery rupture during passage of the device can lead to significant blood loss; if not controlled expeditiously, it can be potentially lethal. Fortunately, with the quality of current computed tomography (CT) imaging, it is possible to anticipate the majority of access problems. Technologic improvements including smaller sizes and hydrophilic coating make endografting accessible to more patients. However, while the delivery profile of abdominal aortic endografts has been decreasing in size, the smallest endograft approved by the U.S. Food and Drug Administration still requires an 18-French sheath, and typically the size requirements range from 20 to 25 French. As such, depending on the device, the iliac arteries should have a minimal luminal diameter of 7 to 9 mm. Apart from diameter, other anatomic factors need to be considered, such as the extent of calcification and tortuosity (Figure 58-1). In general, if only one of these factors is marginal, transfemoral delivery can be attempted. However, if more than one factor is marginal, an alternative access such as an iliac conduit should be considered.
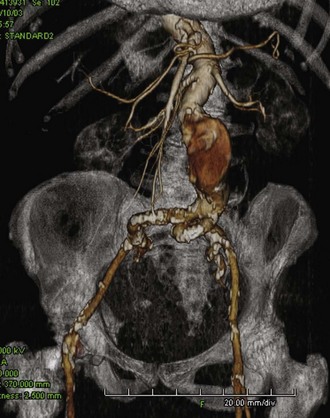
FIGURE 58-1 Challenging iliac access vessels. Note the calcification and severe tortuosity.
(From Moore WS, Ahn SS: Endovascular surgery, ed 4, Philadelphia, 2011, Saunders.)
Two classes of conduits have been described: open and endovascular. Open conduits are typically performed through a retroperitoneal incision. It is the authors’ preference to construct this conduit under combined spinal-epidural anesthesia. A 10-mm crimped Dacron graft is sewn in an end-to-side fashion to the distal common iliac artery, and the graft is clamped distally. The graft can then be punctured and used analogously to a native vessel to allow for delivery of the device. After delivery of the device, the graft can simply be ligated, or it can be tunneled down to the groin and anastomosed to the femoral artery to treat any significant iliac occlusive disease. An open conduit also maintains perfusion to the ipsilateral internal iliac artery (Figure 58-2).
Endoluminal conduits consist of a balloon-expandable stent sewn to a thin-walled, 8-mm PTFE graft. The PTFE is predilated at the tip to an appropriate diameter (the diameter of the common iliac artery at the seal zone), sewn to an appropriately sized balloon-expandable metal stent, and then crimped onto a balloon sized for the iliac seal zone (Figure 58-3A, B). The balloon, stent, and PTFE graft are then back-loaded into a sheath (typically 16 to 18 French), and an angioplasty balloon (typically 6 mm by 2 cm) is used to form a tapered tip for the delivery system. This conduit can be placed using only local anesthesia if necessary. The endoluminal conduit is delivered transfemorally after judicious pre-dilatation of the iliac arteries. Once the stent is in the common iliac artery, the sheath is withdrawn, the stent is expanded, and the PTFE is then dilated to at least 10 mm throughout its entire length down to the groin. At this point, the endoluminal conduit can be accessed in a fashion similar to a native vessel to allow for delivery of the device (see Figure 58-3C, D). Upon completion of the procedure, the PTFE is anastomosed to the femoral artery. The benefit of an endoluminal conduit is that it obviates a retroperitoneal incision in a patient with a hostile abdomen. In addition, in patients with circumferential iliac calcification, the conduit does not need to be sutured to the artery. Disadvantages include the fact that the ipsilateral internal iliac artery needs to be covered. Variations of the endoluminal conduit have been described, such as placement of a commercially available self-expanding stent graft in the iliac artery prior to delivery of the stent graft.10
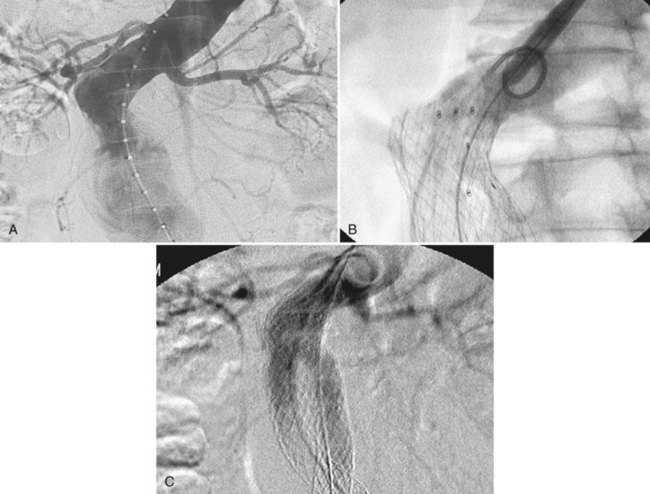
Despite adequate planning, access vessel rupture can occur (Figure 58-4). Two conditions are necessary to allow for a safe outcome. First, prompt recognition of the rupture is mandatory. Any unexplained hypotension intraoperatively should be investigated with a retrograde injection of contrast through the iliac artery. Second, it is absolutely essential to maintain wire access. This allows for placement of a compliant aortic occlusion balloon to control hemorrhage. As the balloon is compliant, it can be inflated in the common iliac artery just proximal to the site of rupture to allow for continued perfusion of the contralateral iliac artery. At this point, a decision can be made as to how to repair the iliac rupture. If the device has already been delivered, an extension limb or a commercially available stent graft can be deployed over the site of rupture. There are several types available (Viabahn, Fluency, Atrium) and the delivery systems range in size from 7 to 11 French. If the rupture is not amenable to repair with a stent-graft, then a retroperitoneal incision can be made in a controlled fashion, and either direct repair of the artery or placement of an open iliac conduit can be performed at this time.
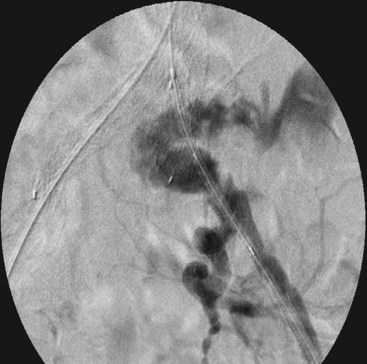
FIGURE 58-4 Retroperitoneal rupture after dilatation of left iliac limb.
(From Moore WS, Ahn SS: Endovascular surgery, ed 4, Philadelphia, 2011, Saunders.)
Acute Neck Complications
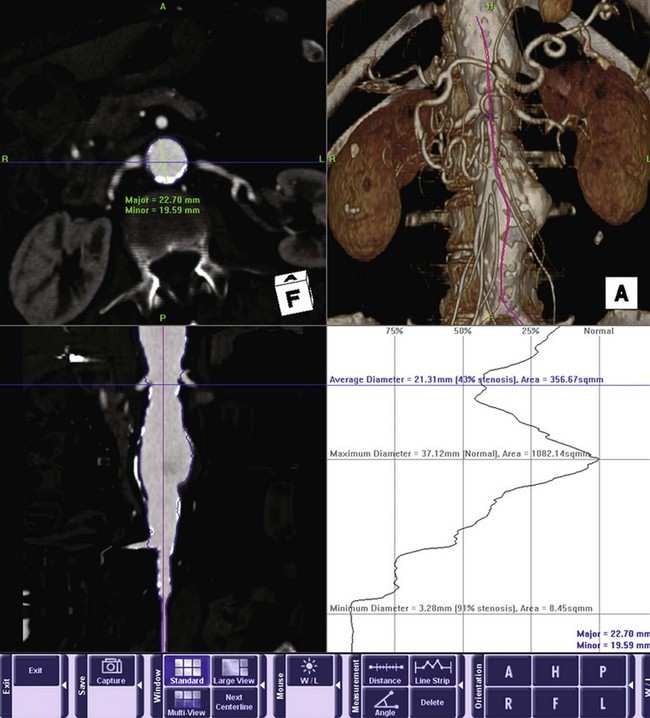
While access vessel issues are responsible for the majority of acute complications, the long-term success of endovascular aneurysm repair (EVAR) is ultimately dependant on the seal zone at the proximal neck. A recent study by AbuRahma and colleagues11 found that aortic neck length of less than 10 mm correlated with an increased rate of both early and late type 1 endoleaks.11 In addition to length, three other anatomic characteristics of the proximal neck determine suitability: angulation, shape, and the extent of mural thrombus (Figure 58-5). In general, long, straight necks with minimal thrombus are ideal. Preoperative assessment with three-dimensional CT angiography is essential in assessing these characteristics. Center-line reformatted images can provide an accurate assessment of neck length, shaded surface reconstructions can give an accurate view of the angulation and shape of the neck, and orthogonal reconstructions allow for accurate diameter measurements and assessment of the extent of mural thrombus. If one anatomic factor is unfavorable, one can consider attempting EVAR. However, if multiple factors are unfavorable, the likelihood of long-term failure increases significantly.12
The choice of device may contribute to the success or failure of the proximal seal zone. Advances in device design such as suprarenal fixation, increased graft flexibility, and an increased range of sizes have allowed endografts to be used in a broader range of patients. However, there are no definitive data proving superiority of any one graft over another.13 The clinician should base their choice of device on several factors, such as diameter, conformability, trackability, and precision of deployment. In addition, the importance of operator familiarity with the device cannot be overemphasized.
Accurate sizing and placement of the device is essential. Angiography from multiple projections should be performed to identify the true origin of the lowest renal artery. Three-dimensional reconstruction technology can allow for optimal determination of gantry angulation prior to the procedure. The entire length of suitable neck should be used to allow for durable fixation of the stent graft. If a proximal type I endoleak is present after placement of the main device, several maneuvers may be helpful. It is the authors’ practice to complete deployment of the contralateral limb before addressing the proximal leak. This provides additional column strength to the device before any salvage maneuvers are undertaken. At this point, the cause of the endoleak needs to be determined. If the device is too low, then placement of an extension cuff is generally the first step. Occasionally, the device abuts the lowest renal but does not cover the entire length of neck on the opposite wall due to neck angulation. In this case, a second cuff may be helpful, as it may position itself in a different fashion once the main body is in place (Figure 58-6).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree