Malignant Pleural Effusions
Steven A. Sahn
Malignant pleural effusions are confirmed by finding malignant cells in pleural fluid or in pleural tissue by percutaneous needle biopsy or at thoracoscopy, thoracotomy, or autopsy. In some patients with established malignancy with an associated pleural effusion, malignant cells cannot be demonstrated in either pleural fluid or pleural tissue and most likely are not present at the time of the diagnostic procedure. Sahn51 labels these effusions “paramalignant” because they are associated with and caused by the malignancy but do not result from pleural invasion by tumor. Paramalignant effusions can be caused by a direct local effect of the tumor, by systemic manifestations of the malignancy, or as a consequence of therapy (Table 71-1). Impaired lymphatic drainage of the pleural space is an important mechanism responsible for the formation of both paramalignant and malignant pleural effusions.
Virtually all cancers can metastasize to the pleura. Lung cancer is the most common to involve the pleura because of its proximity to the pleural surface and, as Meyer42 suggested, its propensity to invade the pulmonary arteries and embolize to the visceral pleura. Breast cancer also frequently metastasizes to the pleura, causing approximately 25% of malignant pleural effusions in large series. Ovarian and gastric carcinomas are next in frequency, and each represents less than 5% of malignant pleural effusions. Sahn52 has found that approximately 7% of patients with malignant pleural effusions have an unknown primary site at the time of the initial diagnosis of the malignant effusion.52
Sahn has noted that lymphomas account for approximately 10% of all malignant pleural effusions52 and, according to Valentine and Raffin,64 are the most common cause of chylothorax. Both Hodgkin’s and non-Hodgkin’s lymphomas have been associated with pleural effusions with variable incidences and usually through different mechanisms (see below).
Diffuse malignant mesothelioma arises from mesothelial cells or possibly from a precursor cell that is situated in the submesothelial connective tissue. The association of asbestos exposure and malignant mesothelioma was established in 1960 by the report of Wagner and colleagues.65 McDonald and coworkers39 recorded that the incidence of malignant mesothelioma is approximately one per million per year in the general population that is not exposed to asbestos. McDonald and associates39 reported that the incidence can rise 20-fold in certain populations and is even higher in shipyard communities.
Pathogenesis
Impaired lymphatic drainage of the pleural space is an important mechanism responsible for accumulation of large volumes of pleural fluid in malignancy. The lymphatic system can be blocked at any point from the stoma of the parietal pleura to the mediastinal and parasternal (internal mammary) lymph nodes. The autopsy studies by Meyer42 and Chernow and Sahn6 have demonstrated the association of mediastinal lymph node involvement and the presence of substantial pleural fluid. Conversely, these studies showed evidence of pleural involvement with tumor in the absence of pleural effusions, lending support to this mechanism. Furthermore, as Meyer42 noted, pleural effusions usually do not occur when the pleura is involved by sarcoma because of the absence of lymphatic metastasis. Weick and associates67 noted that Hodgkin’s disease tends to cause pleural effusions by lymphatic obstruction, and Jenkins and colleagues27 and Xaubet and associates71 noted that non-Hodgkin’s lymphoma tends to produce effusions by both lymphatic obstruction and direct pleural invasion.
The inflammatory response to pleural tumor invasion results in increased microvascular permeability and produces variable volumes of pleural effusion. Chretien and Jaubert7 suggested that oxygen radicals, arachidonic acid metabolites, proteases, lymphocytes, and immune complexes are probably causative.
Vascular endothelial growth factor (VEGF) has been shown to play an important role in the formation of pleural effusions.17 The cytokine VEGF has been documented to induce vascular leakage in the formation of both pleural effusion and ascites. Tumor cells implanted in the pleura of experimental animals secrete VEGF, leading to microvascular permeability. Blocking the VEGF receptor has inhibited pleural fluid formation in an in vivo model of adenocarcinoma of the lung. Mesothelial cells appear to be an important source of VEGF, and transforming growth factor beta has been shown to increase VEGF production both in vivo and in vitro.32
Pleural effusion is an early manifestation of a malignant mesothelioma and probably results from a combination of increased capillary permeability from direct pleural invasion and impaired lymphatic drainage of the pleural space. As the tumor progresses and the visceral and parietal pleura fuse, the fluid diminishes or disappears.
Table 71-1 Causes of Paramalignant Pleural Effusions | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
Autopsy series have shown that in patients with carcinoma of the lung, pleural metastasis is virtually always found on both the visceral and parietal pleural surfaces. Meyer42 noted that rarely is only the visceral pleural surface involved, and isolated parietal pleural metastases were never identified. Visceral pleural metastasis in lung cancer appears to result from contiguous spread or pulmonary arterial invasion and embolization. Once seeded with tumor, these malignant cells migrate from the visceral to parietal pleural surface along either preformed or tumor-induced pleural adhesions. Alternatively, free tumor cells exfoliated from the visceral pleural surface can adhere to the parietal pleura and multiply. Chernow and Sahn6 reported that adenocarcinoma of the lung is the most common cell type to involve the pleura, presumably owing to its peripheral location and propensity for vascular invasion. When bilateral pleural metastases develop in lung cancer, hepatic spread and parenchymal invasion in the contralateral lung usually are causative. Once contralateral lung metastasis occurs, pulmonary artery invasion and embolization follow, as in the initial ipsilateral lesion. The data concerning the laterality of the pleural effusion in relation to the primary lesion support this mechanism.
Chernow and Sahn6 pointed out that in lung cancer, pleural effusions occur either ipsilaterally or bilaterally and virtually never occur solely in the contralateral pleural space. With other cancers, pleural involvement is usually from tertiary spread from established liver metastases with no predilection for side. Fentiman and associates16 summarized the conflicting data in breast carcinoma, with some studies showing a high incidence of ipsilateral pleural effusion and others no predilection for side. Probably two mechanisms are operative: chest wall lymphatic invasion resulting in ipsilateral effusion, and hepatic spread with bilateral, unilateral, or contralateral hematogenous metastasis.
Clinical Presentation
The most common presenting symptom of patients with carcinoma or lymphoma of the pleura and a large pleural effusion is dyspnea with exertion. In diffuse pleural mesothelioma, patients generally present with the insidious onset of either chest pain or dyspnea. Taryle and colleagues62 noted that almost all patients with a malignant mesothelioma present with some symptoms, whereas Chernow and Sahn6 and Weick and associates67 reported that up to 25% of patients with carcinoma or lymphoma of the pleura, respectively, may be relatively asymptomatic when the pleural effusion is initially discovered on a routine chest radiograph.
Because malignant involvement of the pleura signals advanced disease, these patients often have weight loss and appear chronically ill. Chernow and Sahn6 found that a positive pleural cytology provided the initial diagnosis of cancer in almost 50% of these patients, although it is suspected that this percentage would be substantially lower currently due to more sensitive diagnostic techniques.
Patients with carcinoma of the pleura may have chest pain caused by involvement of the parietal pleura, ribs, or chest wall. Elmes and Simpson13 emphasized, however, that the chest pain associated with malignant mesothelioma is more common and impressive but is nonpleuritic and frequently referred to the upper abdomen or shoulder.
Chernow and Sahn6 noted that signs of a pleural effusion are typically found on physical examination and cachexia and lymphadenopathy may be seen in cancer, but the examination may be unremarkable in malignant mesothelioma except for the findings of a moderate to large pleural effusion.
Radiographs of the Chest
The pleural effusion associated with lung cancer is ipsilateral to the primary lesion because of direct pleural involvement, ipsilateral mediastinal lymph node infiltration, or an endobronchial lesion with pneumonia or atelectasis. With other primary sites with the possible exception of breast cancer, there appears to be no ipsilateral predilection and bilateral effusions are common and, as Chernow and Sahn6 have noted, are usually the result of bilateral mediastinal lymph node metastasis; bilateral parenchymal metastasis, thoracic duct involvement, and malignant ascites can also cause bilateral malignant effusions.
Patients with carcinoma of the pleura usually present with a moderate to large effusion (500–2,000 mL); 10% have effusions <500 mL, and a similar number have massive pleural effusions
with complete opacification of the hemithorax. Malignancy is the most common cause of a massive pleural effusion. In a series by Maher and Berger,40 67% of 46 massive pleural effusions were caused by malignancy (Fig. 71-1). The radiographic finding of bilateral effusions with a normal heart size also suggests malignancy, most commonly carcinoma, which Rabin and Blackman46 noted (Fig. 71-2). Nonmalignant effusions associated with bilateral effusions with a normal heart size include lupus pleuritis, esophageal rupture, hepatic hydrothorax, nephrotic syndrome, and constrictive pericarditis.
with complete opacification of the hemithorax. Malignancy is the most common cause of a massive pleural effusion. In a series by Maher and Berger,40 67% of 46 massive pleural effusions were caused by malignancy (Fig. 71-1). The radiographic finding of bilateral effusions with a normal heart size also suggests malignancy, most commonly carcinoma, which Rabin and Blackman46 noted (Fig. 71-2). Nonmalignant effusions associated with bilateral effusions with a normal heart size include lupus pleuritis, esophageal rupture, hepatic hydrothorax, nephrotic syndrome, and constrictive pericarditis.
When a patient presents with an apparent large pleural effusion with absence of contralateral mediastinal shift, or ipsilateral shift, malignancy is usually the cause. The following diagnoses should be considered in this context: (a) carcinoma of the ipsilateral mainstem bronchus causing atelectasis, (b) a fixed mediastinum caused by malignant lymph nodes, (c) malignant mesothelioma (the density represents mostly tumor with a small effusion), and (d) extensive tumor infiltration of the ipsilateral lung radiographically mimicking a large effusion.
As Whitcomb and associates69 and MacDonald38 described in Hodgkin’s disease, patients with pleural effusions usually have associated lymphadenopathy and parenchymal infiltrates. In contrast, Jenkins and colleagues27 reported that in non-Hodgkin’s lymphoma, intrathoracic lymphadenopathy occurs in few of the cases associated with either pulmonary disease or pleural effusions.
Heller and colleagues23 noted that the initial chest radiograph in malignant mesothelioma usually shows a moderate to large unilateral pleural effusion. After therapeutic thoracentesis, the pleura may show thickening and nodularity. Evidence of asbestos exposure, such as interstitial lung disease or pleural plaques, may be identified in the contralateral lung and pleura. Radiographic clues suggesting that the large effusion may be caused by mesothelioma rather than carcinoma are pleural nodularity, absence of contralateral mediastinal shift with an apparent large effusion, and a tendency for loculation.
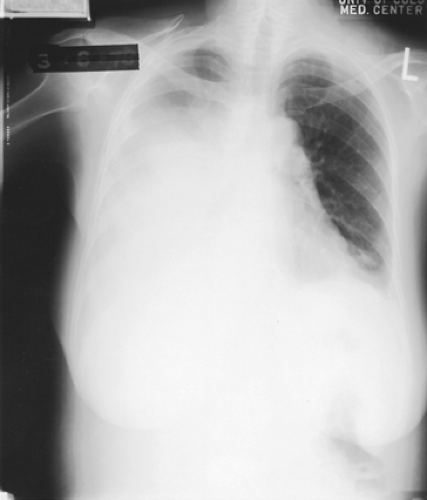 Figure 71-1. A 60-year-old woman with adenocarcinoma of the lung with a massive right pleural effusion. Note the contralateral mediastinal shift. |
Characteristics of Pleural Fluid
Malignant pleural effusions may be serous, serosanguineous, or grossly bloody. A grossly bloody effusion suggests direct pleural involvement, whereas a serous effusion results from either lymphatic obstruction or an endobronchial lesion with atelectasis. Light and coworkers33 suggested that when the red blood cell count in the pleural fluid is greater than 100,000/μL in the absence of trauma, malignancy is the most likely diagnosis. Most of the nucleated cells (2,500 to 4,000/μL) in pleural fluid, as Yam72 noted, are lymphocytes, macrophages, and mesothelial cells; more than 50% of the cellular population are lymphocytes in approximately half the cases. Lymphomatous pleural effusions typically have lymphocyte counts >80%. The percentage of neutrophils usually is <25% of the cell population, but on rare occasions, when there is intense pleural inflammation, neutrophils may predominate. In a prospective study, Rubins and Rubins50 reported that pleural fluid eosinophilia occurred in 7.8% (10 of 128) of patients with malignant effusions and that malignancy is as prevalent among eosinophilic as among noneosinophilic effusions.
Carcinomatous pleural effusions typically are exudative; however, the protein concentration is variable. Chernow and Sahn6
and Light and associates34 reported protein concentrations ranging from 1.5 to 8.0 g/dL. Up to 5% of malignant pleural effusions are transudates. These transudative malignant effusions are caused by early stages of lymphatic obstruction, atelectasis from bronchial obstruction, or most often by a concomitant disease, such as congestive heart failure. With a discordant exudate by lactate dehydrogenase (LDH) criterion only, Light and associates34 emphasized that malignancy should be suspected. Sahn and Good55 found that approximately one-third of patients with malignant pleural effusions have a low pleural fluid pH (<7.30, range of 6.95–7.29) and a low glucose concentration (<60 mg/dL or ratio of pleural fluid to serum of <0.5) at presentation. These effusions usually have been present for a few months and are associated with a large tumor burden and possibly fibrosis of the pleural surface. Good and colleagues20 suggested that the abnormal pleural membrane reduces glucose entry into the pleural space and impairs glucose end-product (CO2 and lactic acid) efflux, resulting in a local acidosis. Furthermore, Sahn and Good55 noted that low pH–low glucose malignant effusions are associated with shorter survival, higher diagnostic yield on initial cytologic examination, and a poorer response to chemical pleurodesis compared with normal pH and glucose malignant effusions.
and Light and associates34 reported protein concentrations ranging from 1.5 to 8.0 g/dL. Up to 5% of malignant pleural effusions are transudates. These transudative malignant effusions are caused by early stages of lymphatic obstruction, atelectasis from bronchial obstruction, or most often by a concomitant disease, such as congestive heart failure. With a discordant exudate by lactate dehydrogenase (LDH) criterion only, Light and associates34 emphasized that malignancy should be suspected. Sahn and Good55 found that approximately one-third of patients with malignant pleural effusions have a low pleural fluid pH (<7.30, range of 6.95–7.29) and a low glucose concentration (<60 mg/dL or ratio of pleural fluid to serum of <0.5) at presentation. These effusions usually have been present for a few months and are associated with a large tumor burden and possibly fibrosis of the pleural surface. Good and colleagues20 suggested that the abnormal pleural membrane reduces glucose entry into the pleural space and impairs glucose end-product (CO2 and lactic acid) efflux, resulting in a local acidosis. Furthermore, Sahn and Good55 noted that low pH–low glucose malignant effusions are associated with shorter survival, higher diagnostic yield on initial cytologic examination, and a poorer response to chemical pleurodesis compared with normal pH and glucose malignant effusions.
Pleural effusions caused by lymphoma have characteristics similar to those of carcinoma of the pleura. These effusions, however, tend to be less hemorrhagic and less likely to result in pleural fluid acidosis and low glucose concentrations. Both Sahn51 and Gottehrer and colleagues21 have pointed out that a pleural effusion in malignant mesothelioma is more likely to have a low pH and low glucose content and greater protein and LDH concentrations than effusions from carcinoma of the pleura. Because of an overlap of values, however, these data are not helpful in separating carcinoma from mesothelioma in an individual patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



