CHAPTER 30 Malignant Pleural and Pericardial Effusions
MALIGNANT PLEURAL EFFUSIONS
Pleural effusions are common clinical problems, occurring in more than 1 million patients each year.1 In some settings, up to 22% of these effusions are caused by malignant disease, and more than 100,000 malignant effusions require treatment annually.2 Affected patients with advanced neoplastic disease experience considerable morbidity as a result of these pleural fluid collections.
Demographics
In adults, 95% of neoplastic pleural effusions arise from a metastatic source, with lung and breast carcinoma accounting for 75% of all cases.3 Other common causes are lymphoma, gastric cancer, and ovarian cancer. About half of patients with breast cancer develop a pleural effusion within their lifetime, compared with one fourth of patients with lung cancer and one third of patients with lymphoma.
Most pediatric effusions, on the other hand, are benign. If malignant, lymphomas or leukemias account for half, with the remainder a mix of tumors such as neuroblastoma, Wilms’ tumor, and germ cell neoplasms.4
Adenocarcinomas whose primary is unknown constitute a distinct entity in patients in whom the source of the pleural malignancy is never found. They are associated with exposure to environmental tobacco smoke.5
Evaluation
Imaging
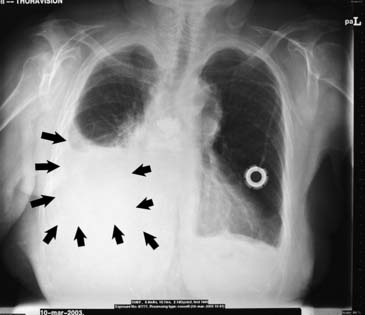
Early chest radiographic evidence of pleural effusion is costodiaphragmatic sulcus blunting caused by as little as 125 to 250 mL of fluid, depending on the quality of the film (Fig. 30-1). Occasionally, a spurlike shadow projects into the fissure. Massive effusions are uncommon, but when they occur they are likely to be malignant. In patients in whom the finding is uncertain, a decubitus film might show shifting of the fluid. Effusions wider than 10 mm by decubitus film are often successfully tapped.6
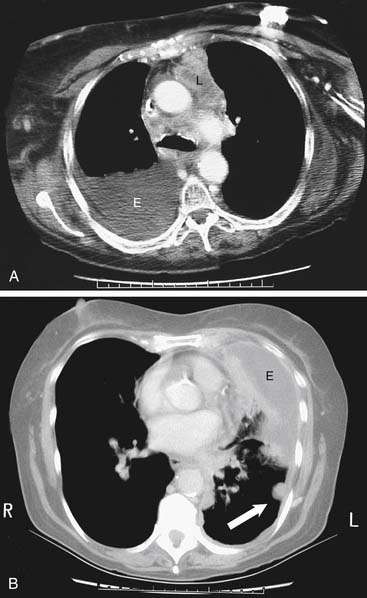
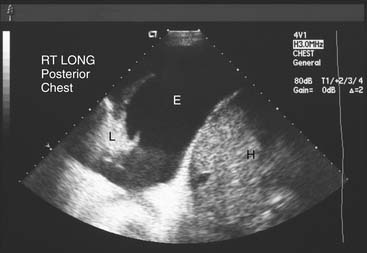
There are specific computed tomography (CT) criteria for pleural effusions. In general, loculation, pleural thickening, pleural nodules, and extrapleural fat of increased density are present only in exudative effusions. Multiple pleural nodules and nodular pleural thickening are generally limited to effusions of malignant etiology (Fig. 30-2). Pleural thickening greater than 1 cm is also a reliable criterion.7 Magnetic resonance imaging (MRI) has limited use, but it may be slightly more useful for certain pleural tumors such as lipoma, and it also can be helpful in determining the extent of invasion for mesothelioma.8 Thoracic ultrasound shows the optimal site for diagnostic thoracentesis of a small pleural effusion. It also helps select ideal entry points for thoracoscopes or pleural biopsy instruments by avoiding adhesions.9
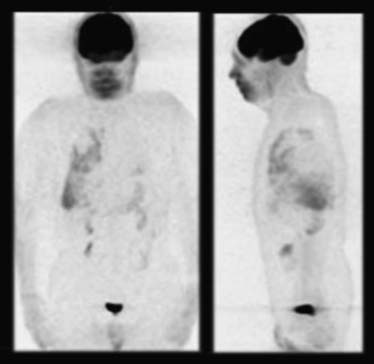
Positron emission tomography (PET)-CT scans may detect early pleural metastases before other imaging tests. If nuclear imaging reveals pleural activity and pleural fluid is scant, diagnostic thoracoscopy is necessary to exclude pleural disease if this staging is relevant. Limited data suggest a high degree of diagnostic accuracy in differentiating benign from malignant pleural effusions.10 Figure 30-3 is an image of a PET scan demonstrating a malignant pleural effusion.
Diagnostic Procedures
Thoracentesis
The diagnosis of pleural effusion is confirmed by thoracentesis. Withdrawing only a small portion of pleural fluid is indicated occasionally when full lung reexpansion is unlikely, or as a prelude to a definitive drainage procedure. It is usually preferable to attempt drainage of as much pleural fluid as possible (Fig. 30-4).
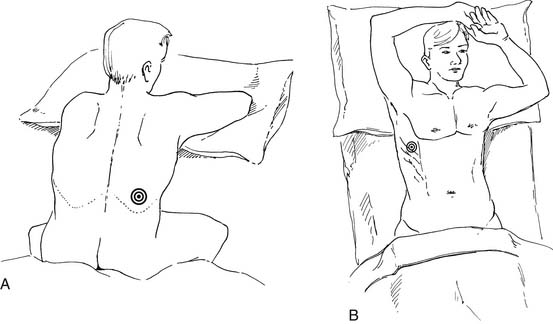
Several commercial kits facilitate drainage by allowing insertion of soft catheters with side holes to completely evacuate the fluid. The catheters can be readjusted during drainage if the lung temporarily occludes the holes. Needles with retractable blunt obturators (e.g., a Turkel needle) also prevent lung injury during pleural entry. Physical examination can guide a pleural tap. On the other hand, in more complex situations, ultrasound or other imaging can optimize needle or chest tube placement for effusion drainage (Fig. 30-5). This is particularly useful for a small effusion or pleural disease of long duration complicated by lung consolidation or pleural loculation. Marking of the area of “deepest” fluid is useful, but it is important to perform the thoracentesis with the patient in the same position as during the imaging. Attempts to completely aspirate chronic effusions should be undertaken carefully. High vacuum applied to the pleural space, by either syringe aspiration or vacuum bottle, is sufficient to rupture alveoli and cause a complicated hydropneumothorax.
Standard Chemistry and Cell Counts
Practically all malignant pleural effusions are exudates. Laboratory criteria to establish exudative effusions are based on absolute values or on ratios to systemic parameters. One of the most useful sets of criteria to classify effusions as exudative was described by Light, as follows1,11:
Low glucose (<60 mg/dL) and pH (<7.20) levels are common in malignant pleural effusions.12 This is attributed to glucose use and acid production by the malignant cells, leukocytes within the pleural fluid, and increased pleural membrane metabolism. Investigators have also implicated abnormal pleural membrane transport of glucose, carbon dioxide, or hydrogen ion.13 Pleural fluid amylase is elevated in about 10% of patients with malignant pleural effusions even without pancreas gland disease.14 In fact, the most common cause of an amylase-rich pleural effusion is neoplasm.15 The cell count can also indicate malignancy. For some investigators, bloody fluid is the strongest positive predictor of malignant effusion. Fluid viscosity has also been studied as a diagnostic test.16
Immunocytochemistry and Special Chemistry
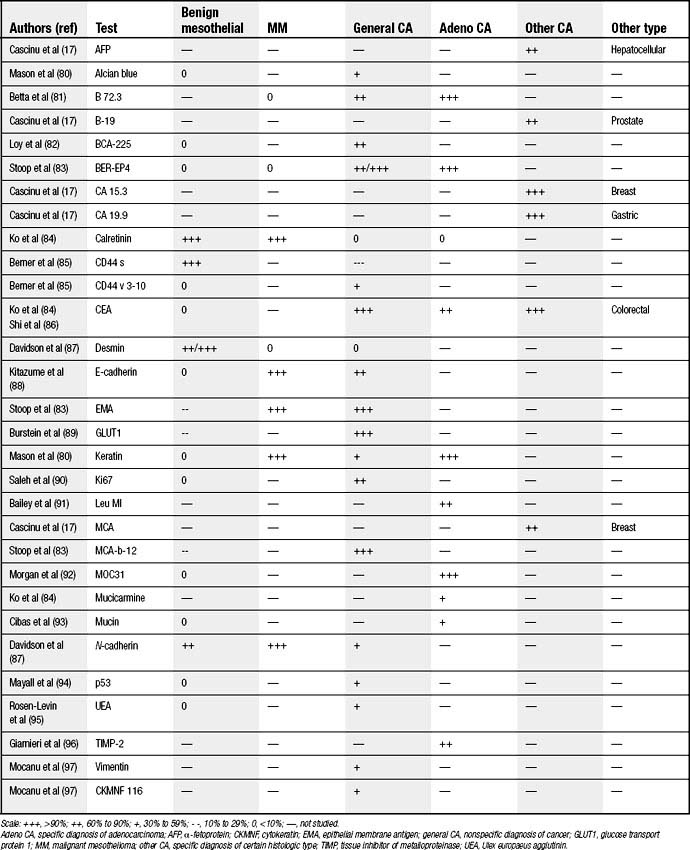
Many investigators focused their explorations on staining patterns of cells obtained from pleural fluid to confirm malignancy and, if present, to correctly classify its origin. Unfortunately, markers are not yet sufficiently sensitive and specific, but they generally show twice the positivity of cytology alone (80% versus 40%).17 These tests are usually used after standard cytologic screening and also are combined with cytogenetic and other miscellaneous tests to establish a diagnosis. A more complete list is displayed in Table 30-1.
Some of these markers, such as p53, predict a worse prognosis for malignant effusions that are p53 negative.18 Another marker, Ki-67, is associated with a worse prognosis when its labeling index is low.19
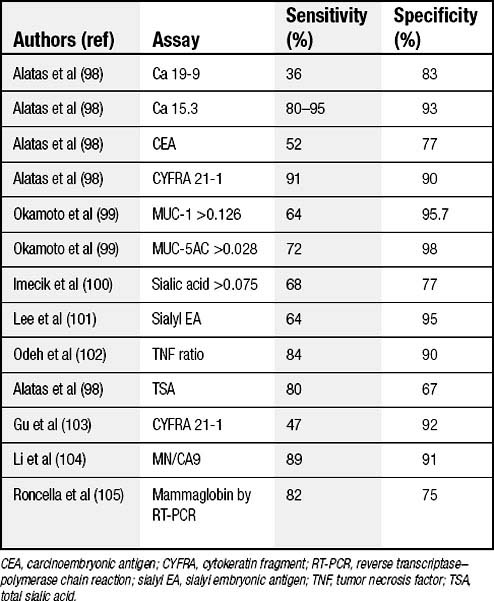
Vascular endothelial growth factor (VEGF) has been found to be a useful discriminator for malignant effusions.20 Other special chemistry values (Table 30-2) are occasionally useful to categorize malignancy. The sensitivities and specificities vary by specific cell type.
Cytogenetics
DNA testing by flow cytometry or chromosome analysis predicts the likelihood of malignancy. The evidence of a marker chromosome, aneuploidy, or a hyperdiploid state suggests malignancy. Aneuploid samples (by flow cytometry) yield predictive values as high as 96%.21 No aneuploidy was found in benign reactive effusions in one series.22 Telomerase activity occurs in 92% of malignant effusions and in only 6% of benign effusions (with a specificity of 94.2%).23
Pleural Cytology and Biopsy
Cytologic evaluations with standard staining (Papanicolaou smears) can sometimes confirm malignant pleural effusions. In very unusual cases (0.5%), the results are falsely positive.24 In cytologic specimen reviews, lung adenocarcinoma is the most frequent diagnosis. Breast cancer effusion specimens have a higher cytology diagnostic yield (about 78%) than lung or other tissue primaries.25 The sensitivity of cytologic evaluation of pleural fluid is frequently greater than that of undirected pleural biopsy.
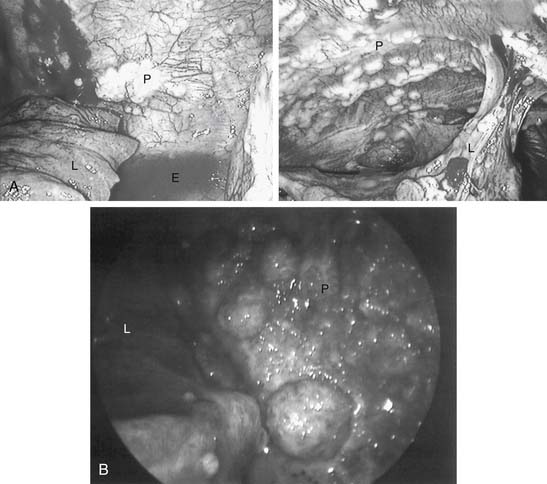
When standard pleural effusion cytology is nondiagnostic, pleural needle biopsy occasionally provides some additional useful diagnostic information (48% in one series).26 However, whether pleural biopsy adds much beyond fluid cytologic evaluation in cases of suspected malignancy is controversial. In another series, only 7% of patients had a diagnosis made by pleural biopsy when the cytology result was negative.27 For difficult cases, cytology combined with needle biopsy results are inferior to those obtained by video-assisted thoracic surgery (VATS) (41% versus 97%).28 In several series, a typical yield of undirected pleural needle biopsy of 50% to 60% was far inferior to the yield of biopsies obtained by VATS. A directed VATS approach in these cases achieves greater than 90% success.29 With idiopathic effusions (20% in some series), uncertainty is reduced to 4% by using thoracoscopy. The complication rate from thoracoscopic biopsy is low, with mortality rates less than 1% and complication rates less than 10%.30 Figure 30-6 shows thoracoscopic images that confirmed pleural malignancy.
A technique variation that might shift results of “blind” biopsy toward those of directed biopsy is the use of a pleural brush to increase the cytologic yield. In one series, this technique was positive in 90% of the cases, as opposed to 67% with the routine cytologic aspirate and 58% with biopsy.31 Thoracotomy to perform pleural biopsy is unusual given the successful directed methods such as VATS.
When patients remain without a definitive diagnosis after VATS or thoracotomy, about one third demonstrate a cause (typically lymphoma or mesothelioma) months to years later.32 For patients with massive malignant effusions of uncertain etiology, fiberoptic bronchoscopy may establish lung cancer. When effusions are mild to moderate and without associated symptoms, the chance of finding a neoplasm is not great enough to warrant bronchoscopy.
Treatment
Treatment options can be classified into those that rely on drainage alone and those that also obliterate the pleural space. As there is an inflammatory response associated with most pleural effusions, persistent and complete pleural drainage may also achieve pleural fusion. evidence shows cytokine activation from repetitive pleural draining that supports this view.33 Although pleural malignancy traditionally means surgical incurability, some investigational multidisciplinary methods include operations, for selected cases, that are designed to improve the chance for cure.34
This imaging appearance generally resolves by 1 to 3 weeks. However, to avoid this phenomenon, some physicians wait for effusion drainage rates to decrease before instilling pleurodesis agents. It is not clear whether this practice is necessary. Long delays before pleurodesis may simply reduce the thoracic cavity area to which the instilled agent is exposed when significant pleural adhesions have already occurred in regions remote to the drainage catheter. Occasionally, significant pleural thickening or even fibrothoraxes occur as long-term sequelae of pleurodesis. The use of thoracic ultrasound (see Fig. 30-5) can guide the optimal drainage site for small pleural effusions or the optimal point of entry of a thoracoscope to avoid adhesions.
Drainage-Based Methods of Malignant Effusion Control
Small Catheter Drainage
Implantation of a long-term pleural catheter or percutaneous medium-durability pleural catheter placement for continuous or intermittent drainage is better than serial percutaneous aspirations.35 The former methods have the advantage of obtaining nearly complete removal of the pleural fluid in the same way that a tube thoracostomy evacuates the pleural space. Needle aspiration rarely evacuates the fluid completely and rarely maintains the lung expansion long enough for a spontaneous pleurodesis to occur. Even without introduction of any pleural sclerosing agents, pleurodesis occurs simply by maintaining pleural apposition, in a select group of patients, probably from the underlying inflammation and invasive effects of the neoplasms.36
Using a small silicone catheter (Fig. 30-7) with a valve that allows drainage only when connected to a closed system, similar success at 30 days has been achieved in prospective randomized clinical trials compared with traditional pleurodesis methods.
This research was replicated with the Tenckhoff catheter, the pigtail catheter, and the Pleuracan device (Braun, Helsungen, Germany).37–39 All controlled dyspnea in greater than 90% of the cases. Intermittent pleural drainage is an attractive option for patients in whom pleurodesis is less likely to occur because of the inability of the visceral pleura to touch the parietal pleura. As a consequence, this method is often used to complement sclerosis options at various centers.
Pleuroperitoneal Shunting
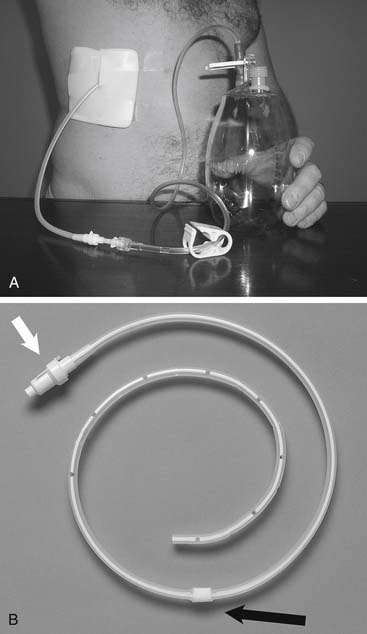
Another way to drain pleural fluid is to pump it into the peritoneal cavity using a device similar to that crafted to drain ascites into the venous system. This generally requires the patient to pump the shunt chamber (Fig. 30-8) at least 20 times four times a day, and perhaps more because of the need to overcome the negative intrapleural pressure.40
Figure 30–8 Pleuroperitoneal shunt. A, Diagram of shunt position and demonstration of proximal and distal obstruction. B, Roentgenogram of lower chest and upper abdomen, showing left pleuroperitoneal shunt (arrows) with chamber placed at costal margin for patient self-pumping. C, CT image showing the catheter (arrows) in the left lower pleural space. This image was obtained 3 months after shunt placement in the patient whose left lower lung loculated pleural breast carcinoma effusion as depicted in Figure 30-2B.
(A, reprinted with permission from Ponn RB, Blancaflor J, D’Agostino RS, Kiernan ME, Toole AL, Stern H. Pleuroperitoneal shunting for intractable pleural effusions. Ann Thorac Surg 1991;51:605-09.)
More than 80% of patients reported excellent results with this method, and it avoided the need for an appliance to traverse the skin. Unfortunately with this technology, at least 10% to 20% of patients occlude their shunt, possibly requiring revision; furthermore, determination of shunt obstruction may be difficult.41 Otherwise, this shunt appears to have survival rates similar to talc pleurodesis. Also, there is generally no evidence that peritoneal deposits result from this pleuroperitoneal shunting technology. The decision to use the shunt can be made during thoracoscopy when pleural coaptation seems unlikely, and it is relatively easy to place the shunt while the patient is under general anesthesia.
Sclerosis-Based Pleural Effusion Management
Generally, accelerating the process of pleurodesis (which might not occur by prolonged drainage alone) is done by accessing the pleural space, draining the majority of fluid, and then inserting a substance that increases the inflammatory response and thus causes intense adhesions within the pleural envelope. There is controversy regarding how pleural fluid characteristics affect the success of pleurodesis and the prognosis of the patient. For example, investigators have shown that a low pleural-fluid glucose level (<60 mg/dL), low pH (<7.2), low performance status (Karnofsky, <70), massive effusion, and high pleural LDH levels (>600 U/L) are associated with a higher rate of failure of pleurodesis efforts. These values also predict positive pleural fluid cytology and a poor overall prognosis.42 However, other investigators have contradicted this finding, particularly with respect to pleural effusion pH.43 Experimental evidence supports the concept that the inflammatory effect needed by some sclerosant agents is inhibited by steroid use, thereby decreasing the effectiveness of the pleurodesis.44
Rolling the patient into different positions after instilling the desired sclerosant is another common practice. This is unnecessary in patients with normal pleural spaces in whom aqueous sclerosing agents were used. Rapid intrapleural dispersion occurs in just one position, as demonstrated by radiopharmaceutical labeling and nuclear medicine imaging.45 However, when the pleural space is loculated or there is lack of full lung expansion, rolling may be useful. Sclerosing agents that are particulate, such as talc slurry, might achieve more uniform distribution with rolling, but this has been disproved in one small prospective trial.46 There also appear to be variations in the practice of pleurodesis, with some surgeons more likely to use an agent such as talc and other physicians using bleomycin or cycline-based drugs.
Another controversy is whether the pleural effusion drainage rate should be tapered before instilling a sclerosing agent. This may be unnecessary. Investigators have shown that daily drainage of pleural fluid is much less useful than achieving full pleural fluid evacuation and lung reexpansion on chest roentgenogram.47 Pleurodesis appears to have only a minor adverse effect on respiratory function, although the studies on this are limited.48 Finally, whereas sclerosis of the pleural cavity is generally practiced on inpatients, there have been successful outpatient pleurodesis programs.
The trend has been toward the use of smaller catheters, both for drainage and for the pleurodesis of malignant pleural effusions. In a prospective randomized study, a 12-Fr catheter was comparable to a standard large bore tube.49 This has been reproduced in other studies of both inpatient and outpatient pleurodeses using the pigtail, Cystofix (Braun), Elecath (Electro-Catheter, Rahway, NJ), and PleurX (Denver Biomedical, Golden, CO) catheters and talc, doxycycline, or similar traditional sclerosing agents.50–53
Techniques of Sclerosis
Chemical Sclerosis: Talc
Talc was first used to create pleural adhesions for tuberculosis management in 1935, and about 25 years later it started to become popular for controlling malignant effusions. Generally, there are two ways to administer talc into the pleural space. One is as an insufflated powder (talc poudrage) and the other is by instillation of a particulate slurry, with the talc in a liquid vehicle. The amount of talc needed depends somewhat on the route of delivery and its preparation method. A fine powder from an aerosol gives a broader distribution with less mass (Fig. 30-9). Nevertheless, reported successful talc pleurodeses generally used between 2 and 8 g, with 5 g being the most common amount used in multicenter trials.
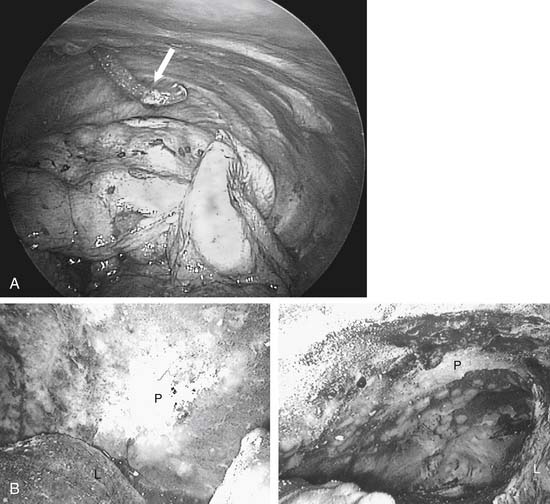
Figure 30–9 A, Thoracoscopic image of left chest before insufflation of talc. Angled tip on insufflation catheter (arrow) facilitates directing talc to desired areas. B, Thoracoscopic image of left chest after insufflation of talc (see same patient, Fig. 30-6A). L, lung; P, parietal pleura.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree