Malignant Mesothelioma and Other Primary Pleural Tumors
The pleura is a membranous structure covering the entire surface of the lung and lining the inside of the chest cavity. It is composed of a thin mesothelial layer with underlying fibroblasts and varying amounts of collagenous fibrous tissue with interdigitating capillaries and venules. The most common tumors of the pleura are metastatic neoplasms, predominantly of lung, breast, or colonic origin. Tumors arising primarily from the pleura are rare, but still constitute a variety of benign and malignant lesions from several different cells of origin, some of which have yet to be identified.
MALIGNANT MESOTHELIOMA
The most common primary malignant tumor of the pleura is malignant mesothelioma, an insidious neoplasm with a generally dismal prognosis arising from the mesothelial surfaces of the pleural and peritoneal cavities, as well as from the tunica vaginalis and pericardium. Eighty percent of all cases of mesothelioma are pleural in origin.
 EPIDEMIOLOGY
EPIDEMIOLOGY
The incidence of mesothelioma in the United States is estimated to be 3300 cases per year.1 The incidence within the United States peaked in the year 2000 and is now declining, secondary to control of asbestos exposure.2 However, incidence is increasing in other parts of the world, such as Europe, Japan, and Australia. In Great Britain, mesothelioma death rates rose from 153 per year in 1968 to 1848 per year in 2001. Similar numbers of deaths are expected annually until the year 2015 when a peak of up to 2450 deaths per year is expected.3 After that time, mesothelioma rates are expected to drop in England and other developed countries because of legislation aimed at reducing asbestos exposure in the workplace and the general environment. In contrast, mesothelioma incidence rates are predicted to escalate for much longer times in the Third World because of poor regulation of asbestos mining and widespread industrial and household utilization of asbestos.3–5
 ETIOLOGY
ETIOLOGY
Inhalational exposure to asbestos has been clearly established as the predominant cause of malignant mesothelioma in humans. Approximately 70% of cases of pleural mesothelioma are associated with documented asbestos exposure. In ancient Greece, the philosopher Pliny first established the association between asbestos exposure and lung disease by making the observation that slaves working in asbestos mines were less healthy than other slaves. It was not until 1960, with the publication by Wagner et al.6 of a series of 33 mesothelioma cases occurring in a crocidolite mining community in South Africa, that the etiologic connection between asbestos and mesothelioma was established. Wagner’s study was soon followed by several other accounts of mesothelioma afflicting asbestos workers at locations around the world. In addition to asbestos miners and workers, other occupations at especially high risk include plumbers/pipefitters, mechanical engineers, ship and boat building and repairing.4
The lifetime risk of developing mesothelioma among asbestos workers is thought to be as high as 8% to 13%.7 There is a latency period of approximately 30 to 40 years from the time of asbestos exposure to the development of mesothelioma. It remains unclear whether there is a dose–response relationship between asbestos exposure and development of mesothelioma. The possibility of a dose–response relationship was demonstrated in a cohort study of over 4500 people who resided in an Australian city that produced crocidolite asbestos but who did not directly participate in its mining or milling. In this study, the incidence of mesothelioma increased significantly with greater environmental exposure, based upon the neighborhood and duration of residence. In a cohort of textile workers with heavy exposure to asbestos, the risk of pleural mesothelioma was increased and was proportional to the latency period. In contrast, many well-documented cases of mesothelioma occur after brief, but intense, or longer-term low-level exposures to asbestos (i.e., spouses of asbestos workers exposed by washing clothes).8
Asbestos is not a specific compound, but the commercial name for a group of hydrated magnesium silicate fibrous minerals divided into two major types: the serpentines and the amphiboles. Serpentine chrysotile fibers, which account for more than 95% of the asbestos previously used for industrial purposes in the United States, are spiral-shaped, pliable, easily breakable, and soluble in tissues, whereas the amphiboles (crocidolite, amosite, tremolite, anthophyllite, actinolite) are rigid, long and needle-like, with a longer biologic persistence than the serpentine fibers. The carcinogenicity of asbestos is thought to depend on dose, biodurability, surface reactivity, and the physical properties of the fibers. Fibers with a high length-to-width ratio, such as crocidolite, which are able to more readily penetrate through the lung to the pleural surface, are considered more carcinogenic. Among the remaining asbestos fibers, amosite has an intermediate carcinogenic risk, chrysotile the lowest. It is unclear whether the cases of mesothelioma attributed to chrysotile exposure are caused primarily by the chrysotile itself or by contamination with tremolite fibers. All asbestos fiber types, however, have carcinogenic potential.3,4
The development of malignant pleural mesothelioma has also been associated in rare cases with other etiologic factors, including therapeutic ionizing irradiation to supradiaphragmatic fields,9–13 intrapleural thorium dioxide (Thorotrast), and inhalation of other fibrous silicates such as erionite. Epidemiologic studies of a region in central Anatolia (Turkey) with an abnormally high incidence of pleural mesothelioma (22 per 10,000 individuals over 25 years old) implicated routine household use of a locally ubiquitous silicate, erionite, as a potential etiologic agent. However, it appears that only specific families (who appear to have as yet to be defined genetic abnormality) are susceptible to erionite-induced mesothelioma.14
 MOLECULAR PATHOGENESIS
MOLECULAR PATHOGENESIS
The latency period from asbestos exposure to the development of mesothelioma ranges from approximately 20 to 50 years, suggesting the necessity of multiple genetic alterations for eventual malignant transformation of the mesothelium. Despite extensive investigation, the exact mechanisms of asbestos carcinogenesis have not yet been fully elucidated. In rodent model systems, asbestos fibers act like tumor promoters in combination with a carcinogen, eliciting proliferation of mesothelial cells. Asbestos fibers can also interact with the mitotic spindle to cause missegregation of chromosomes and aneuploidy. In rat pleural mesothelial cells, asbestos fibers and erionite have been shown to induce the protooncogenes c-fos and c-jun in a prolonged and dose–responsive manner. Several growth factors, secreted by mesothelial/mesothelioma cells in an autocrine fashion, have been implicated in various stages of mesothelioma tumorigenesis. Platelet-derived growth factors A and B (PDGF A and B), insulin-like growth factors 1 and 2 (IGF-1 and IGF-2), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), hepatocyte growth factor, transforming growth factor-β1, 2, and 3 (TGF-β1, 2, and 3) constitute a complex mixture of autocrine and paracrine stimuli for mesothelioma cell proliferation as well as initiation of tumor angiogenesis. More recently, asbestos has been shown to induce necrotic cell death with resultant release of high mobility group protein B1 (HMGB-1) in the extracellular space, which leads to a chronic inflammatory response involving macrophage accumulation and secretion of tumor necrosis factor α (TNFα). This in turn activates NF-κB leading to survival of human primary mesothelial cells that have accumulated genetic damage from asbestos exposure. There is also evidence implicating aberrant activation of the Wnt signaling pathway in mesothelioma.15,16
It has been well established that chronic inflammation predisposes to cancer development. Asbestos fibers appear to stimulate the production of chronic oxidative stress in lung macrophages and other cells for many years. In animal models, crocidolite fibers clearly induce specific DNA adducts (8-hydroxydeoxyguanosine, 8-OHdG) associated with oxidative damage in the DNA from peritoneal cells and macrophages of asbestos-exposed animals. These same type of 8-OHd DNA adducts have been observed in the blood lymphocytes of asbestos-exposed individuals decades after exposure suggesting chronic exposure to oxidant stress.17
The pathogenesis of malignant mesothelioma involves molecular changes such as chromosomal alterations in tumor suppressor genes, allele loss, gene silencing by DNA methylation in specific chromosomal regions, epigenetic dysregulation of tumor suppressor genes by histone acetyltransferase and histone deacetylase (HDAC) chromatin condensation/decondensation balance, and gene mutations. Analysis of explanted human mesotheliomas and cultured human mesothelioma cell lines has revealed a number of cytogenetic aberrations that may predispose to the development of the malignant phenotype. Partial or total loss of chromosomes 1, 3, and 4, deletions of 9p, and monosomy of chromosome 22 are the most common abnormalities seen. For mesotheliomas, 9p deletions have been associated with the loss of function of the p16INK4 cdk inhibitor, a putative tumor suppressor gene, engendering unchecked cdk4-mediated phosphorylation of the retinoblastoma 1 (Rb1) gene product and leading to loss of regulation of cell division. Monosomy 22, the most frequent numerical cytogenetic abnormality in mesothelioma, has recently been correlated with mutations in the neurofibromatosis 2 (NF2) tumor suppresser gene mutations more commonly associated with acoustic neuromas, schwannomas, and meningiomas. The product of NF2, Merlin, appears to inhibit cell proliferation and cell cycle progression by repressing cyclin D1 expression as well as inhibiting invasiveness. The presence of the Wilms tumor suppresser gene (WT1) in human mesotheliomas raises the possibility that alterations in this gene or binding of the WT1 gene product to the p53 tumor suppressor may predispose to mesothelial cell carcinogenesis.15,16,18
 VIRAL ONCOGENES
VIRAL ONCOGENES
Simian virus-40 (SV-40) is a polyoma virus with oncogenic potential in humans. Its actions are thought to result from inactivation of tumor suppressor genes such as the retinoblastoma gene (Rb) and wild-type p53 (wt p53) by a peptide known as the SV-40 large T-antigen (Tag). SV-40 is a potent oncogenic virus in human and rodent cells; SV-40 DNA sequences have been identified in brain tumors, osteosarcomas, and lymphomas.19 SV-40 is the only agent known to cause malignant transformation of human primary mesothelial cell in vitro.20 When cell lines are exposed to both SV-40 and asbestos fibers, the rate of transformation increases significantly. Animal studies have also shown that crocidolite asbestos and SV-40 can act as cocarcinogens.21 Several studies have documented the presence of SV-40 in patients with mesothelioma (some of whom did not have obvious asbestos exposure), as well as in cases of atypical mesothelial proliferation.22–26 As an example, one report examined 35 archival mesothelioma specimens and found that SV-40–like sequences were present in 86% of cases.23 However, the possibility that technical factors can produce false-positive results suggestive of SV-40 infection also has been raised.27,28 Despite the fact that there was worldwide dissemination of SV-40 contaminated polio vaccines in the 1950s and 1960s, there is no convincing epidemiologic evidence linking SV-40 exposure to the development of malignant mesothelioma.
Nonetheless, it is possible that Tag interference with Rb and wt p53 may play an accessory role in the carcinogenesis of malignant mesothelioma.29 If this hypothesis is validated, novel strategies of vaccination to prevent mesothelioma or molecular techniques to improve early diagnosis may become possible.
 GENETIC PREDISPOSITION
GENETIC PREDISPOSITION
Gene polymorphism studies are in their early stages in asbestos-exposed populations. Some suggestive associations in DNA repair genes with mesothelioma development have been reported, but need validation. One study reported an increased incidence of mesothelioma among asbestos-exposed individuals in Finland found to be lacking the glutathione-S-transferase M1 (GSTM1) gene and carrying the “slow-acetylator” type of the N-acetyltransferase 2 (NAT-2) gene.30 The GSTM1 gene is important in the detoxification of several carcinogens, including polycyclic aromatic hydrocarbons; NAT-2 is associated with the biotransformation of aromatic amines. Some genetically predisposed families have been identified, but without identification of a specific “mesothelioma” gene. An increased frequency of polymorphisms in the nod-like receptor pyrin domain (NLRP) inflammasome complex among a cohort of Italians with mesothelioma has also been described.31 Inactivation of the nuclear deubiquitinase BAP1, which appears to regulate key histones and transcription factors related to the development of tumors, is associated with malignant pleural mesothelioma.32 A so-called BAP1 “syndrome” has been described in some families bearing this gene mutation, in which there is higher frequency of mesothelioma, ocular melanoma, and melanocytic lesions of the skin, sometimes even occurring simultaneously in the same individual.33
 PATHOLOGY
PATHOLOGY
Gross and microscopic pathologic findings in malignant mesothelioma are discussed below.
Gross Pathology
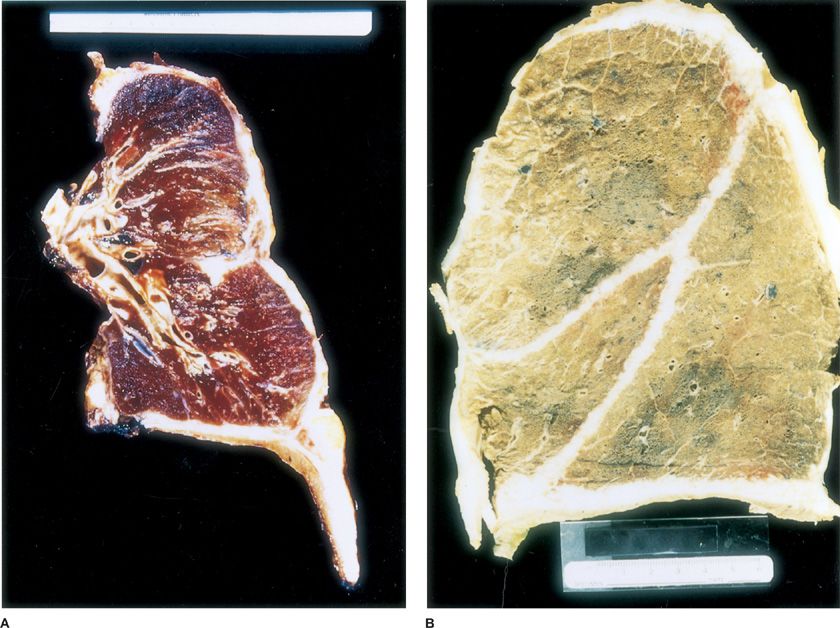
The vast majority of malignant mesotheliomas involving the pleura are those tumors that diffusely involve the pleura and are properly termed “diffuse malignant mesothelioma.” A rare localized gross variant of malignant mesothelioma that forms a single mass arising in the pleura but is otherwise microscopically identical to diffuse malignant mesothelioma has been described and termed “localized malignant mesothelioma.” Diffuse malignant mesothelioma begins as multiple discrete nodules that, in earlier stages, tend to preferentially involve the parietal pleura over the visceral pleura. In time, these nodules tend to coalesce on the visceral and parietal pleural surfaces with subsequent fusion of the pleurae. Progressive tumor growth typically leads to partial or complete encasement of the lung with rinds of pleural tumor that can be several centimeters in thickness, but may show only minimal penetration of the underlying lung parenchyma (Fig. 79-1). Advanced cases show more extensive spread along interlobar fissures, deeper invasion into the underlying lung parenchyma and through the diaphragm, as well as contiguous involvement of the chest wall, pericardium, and mediastinum. Although it is rare for patients with mesothelioma to present clinically with metastatic disease, peribronchial lymphovascular spread, regional lymph node metastases, and extrathoracic hematogenous metastases become increasingly common over the course of the disease. Seventy percent of patients have mediastinal lymph node involvement at autopsy. Hematogenous metastases follow the exact same pattern of spread as non–small cell lung carcinomas with involvement of the contralateral lung and pleura, liver, adrenals, bone, brain, and kidney.
Figure 79-1 A. Transverse section of an extrapleural pneumonectomy surgical specimen with the entire right lung, parietal and visceral pleurae, portions of pericardium, and the majority of the right hemidiaphragm. Note the thick rind of tumor along the pleural surface encasing the lung and invading the diaphragm. B. Postmortem mesothelioma specimen with overnight formalin inflation and fixation. The right lung pictured is covered by a thick, whitish rind of tumor involving the entire pleural surface, which has also infiltrated and demarcated the interlobar fissures.
Histology
The 2004 revision of the WHO classification of pleural tumors recognizes four major histologic subtypes—epithelioid, sarcomatoid, desmoplastic, and biphasic. In the 2004 WHO classification, the use of the term “well-differentiated papillary mesothelioma” is restricted to an exceptionally rare and distinctive mesothelial tumor that has bland cytologic features, stout papillary architecture, and a tendency toward superficial spread without invasion. Part of the diagnostic utility of the WHO classification is that each subtype is associated with a particular differential diagnosis that guides the pathology workup. This workup requires additional time and expense. Multiple sections may be taken and ancillary studies are usually required for definitive diagnosis. From a prognostic perspective, most studies have shown that the purely epithelioid subtype has the longest survival but these differences in survival, on the basis of histologic subtype, are within the range of only a few months. It should be recognized that the larger the tissue sample, the more frequent the histologic variation and the higher the incidence of biphasic tumors.
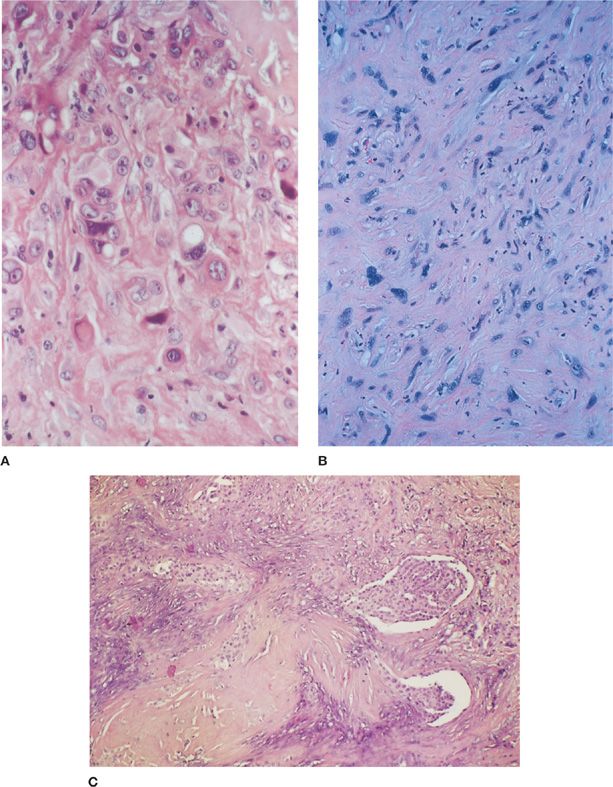
The epithelioid variant is the most common with a wide range and mix of histologic patterns. Typical histologic appearances of this subtype include tubulopapillary, glandular/microglandular, and solid sheet-like patterns (Fig. 79-2A). A myxoid matrix may be prominent and may be mistaken for mucin, but this matrix is actually hyaluronate and shows hyaluronidase-sensitive staining with Alcian blue.
Figure 79-2 A. Photomicrograph of an epithelial malignant mesothelioma. These sheets of pleomorphic cells are epithelial in appearance, with eosinophilic cytoplasm and fairly well-defined cell borders. Note the cytoplasmic vacuoles, which can lead to confusion with a signet ring type of adenocarcinoma. By electron microscopy, these vacuoles can be shown to contain crystallized hyaluronic acid (H&E, ×400). B. Photomicrograph of a sarcomatoid malignant mesothelioma. This tumor has a malignant mesenchymal appearance with bizarre spindled cells and a growth pattern resembling that of a sarcoma. These cells demonstrated strong cytokeratin positivity on immunohistochemical staining, distinguishing this tumor from a sarcoma (H&E, ×400). C. Photomicrograph of a biphasic malignant mesothelioma. This tumor demonstrates several areas of epithelioid histology with a papillary growth pattern seen against a background of spindled and more poorly differentiated epithelioid cells (H&E, ×200).
Sarcomatoid mesotheliomas can also have a wide variety of histologic patterns. The most frequently encountered pattern is that of fibroblastic-like spindle cells arranged in storiform, fascicular, or haphazard patterns that mimic a fibrosarcoma (Fig. 79-2B). Other variants include a malignant fibrous histiocytoma-like tumor and malignant mesotheliomas with malignant smooth muscle, chrondroid, osseous, or rhabdomyoblastic differentiation.
Desmoplastic mesotheliomas, by definition, have areas of densely collagenized tissue with atypical cells arranged in a storiform or “patternless” pattern. This pattern should comprise at least 50% of the tumor. The deceptively bland appearance of the tumor makes its separation from fibrous pleuritis exceedingly difficult, particularly with limited sampling. Studies that have examined the criteria used for diagnosis have highlighted the importance of “interface biopsies” in which unequivocal evidence of invasion into the underlying adipose tissue, skeletal muscle, or lung may be demonstrated. Other criteria, which may require multiple tissue sections to detect, include obvious sarcomatoid areas, foci of necrosis, and distant metastases. Bone metastases similarly may be deceptively bland and confused with a primary benign fibrous tumor of bone.34,35
Biphasic mesotheliomas have both epithelioid and sarcomatoid components (Fig. 79-2C). Each component should represent at least 10% of the tumor for the designation of biphasic. Biphasic mesotheliomas represent about 30% of cases. As previously noted, the percentage of biphasic tumors, which have a prognosis that is intermediate between the epithelioid and sarcomatoid subtypes, increase with larger tumor samples. In recent years, the percentage of sarcomatoid component within biphasic mesotheliomas has been shown to be prognostic factor, with greater than 50% sarcomatoid elements corresponding to decreased overall patient survival.34
Immunohistochemistry
Immunohistochemistry (IHC) has largely replaced electron microscopy as the gold standard for diagnosis. This is because of the comparative low cost, ease, and greater availability of IHC, as well as the expanded array of commercially available antibodies that are reliable markers of mesothelial differentiation. Because there is no single marker with sufficiently high sensitivity and specificity for malignant mesothelioma, it is standard practice for pathologists to employ a panel of markers (both positive and negative) to confirm the diagnosis of malignant mesothelioma. Institutions will vary somewhat in their selection of which markers to include and these panels are typically refined as publications appear with comparative utility studies. As in any instance in which IHC is used as an adjunct in tumor diagnosis, careful consideration must be given to the tumor’s histologic appearance as well as the clinical-radiographic context and the differential diagnosis that is generated from this information.36
Broad-spectrum cytokeratin (CK) antibody cocktails are essential in the diagnosis of malignant mesothelioma. In epithelioid tumors, strong and diffuse CK positivity can be used to exclude the rare case of large cell lymphoma, epithelioid vascular tumors, or melanoma involving the pleura. CK reactivity usually differentiates malignant mesotheliomas from many sarcomas, although there are occasional CK-negative sarcomatoid mesotheliomas as well as focally CK-positive sarcomas. Although CK positivity does not distinguish malignant mesothelioma from reactive lesions, positive CK staining may help to highlight invasion into adjacent structures.
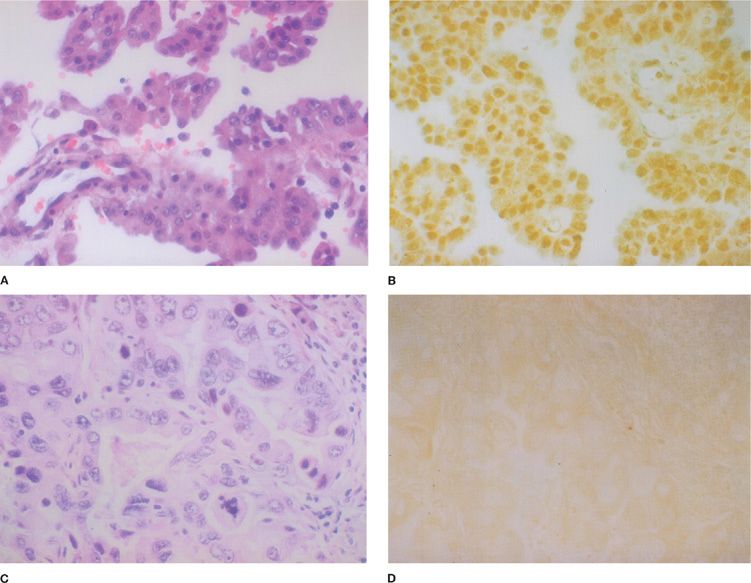
Common affirmative immunohistochemical markers, which, if positive, can be used to support a diagnosis of malignant mesothelioma include calretinin, CK5/6, the Wilms tumor I (WT1) antigen, and D2-40 (Fig. 79-3).36 These markers are most useful in the narrow differential diagnosis of malignant epithelioid mesothelioma versus primary pulmonary adenocarcinoma. It should be noted that these markers do not invariably exclude other tumors, including metastases from nonpulmonary primary sites. A wide variety of markers can be used to support a diagnosis of adenocarcinoma, as opposed to malignant mesothelioma. Markers such as CEA, Leu-M1 (CD15), thyroid transcription factor-1 (TTF-1), Ber-EP4, B72.3, Bg8, and MOC 31 are commonly included in such panels.36 The sensitivity and specificity of both the affirmative mesothelioma markers as well as the adenocarcinoma markers vary greatly when the differential diagnosis is broadened to include other subtypes of primary pulmonary carcinoma such as squamous cell carcinoma or metastases from extrapulmonary sites such as the kidney and ovary. Both categories of markers are generally less reliable in the differential diagnosis of sarcomatoid lesions. The immunohistochemical panel that is recommended for the initial evaluation of a sarcomatoid tumor involving the pleura includes CKs (including AE1/3, CAM5.2, CK18, and CK7), calretinin, and D2-40.37–39 If other types of sarcomas are being considered, then the marker panel should be expanded accordingly to include antibodies such as CD31, CD34, desmin, myoglobin, and S-100.
Figure 79-3 A. Photomicrograph of an epithelial mesothelioma (H&E, ×400). B. Photomicrograph of epithelial mesothelioma demonstrating positive nuclear staining with an antibody to the Wilms tumor 1 (WT1) gene product (×400). C. Photomicrograph of an adenocarcinoma metastatic to the pleura (H&E, ×400). D. Photomicrograph of pleural adenocarcinoma stained with an anti-WT1 antibody (×400). Only minimal background staining is present.
 OTHER ANCILLARY STUDIES
OTHER ANCILLARY STUDIES
Histochemical stains for the presence of intracytoplasmic mucin are still commonly used as a means of differentiating adenocarcinomas from epithelioid malignant mesotheliomas. Mucicarmine and periodic acid-Schiff (PAS) with diastase are the two most frequently used. These stains are technically easy to perform, inexpensive, and rapid. Care must be taken to exclude the possibility of false-positive staining that can be seen with hyaluronate. The use of histochemical staining (Alcian blue with hyaluronidase) to detect the high levels of hyaluronic acid in mesothelioma cells was used far more frequently before the widespread use of IHC.
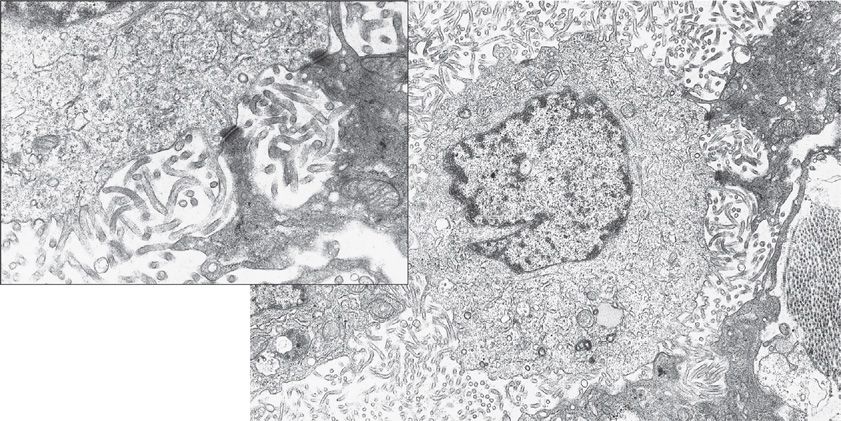
Electron microscopy had traditionally been considered the gold standard for the diagnosis of malignant mesothelioma and ultrastructural analysis can still be useful in occasional problematic cases. The predominant epithelioid form is composed of polygonal cells with numerous long surface microvilli, prominent desmosomes, and abundant tonofilaments (Fig. 79-4). Electron microscopy of the sarcomatoid variant reveals the presence of elongated nuclei, CK and vimentin filaments, as well as copious rough endoplasmic reticulum, some intracellular attachments, and rare microvilli. Electron microscopic studies may be inconclusive in poorly differentiated tumors of either subtype and have no utility in the diagnosis of desmoplastic malignant mesothelioma. Molecular analysis can be performed on formalin-fixed, paraffin-embedded tissue to demonstrate the X:18 translocation characteristic of synovial sarcoma—a biphasic or monophasic sarcomatoid tumor that can involve the pleura. As discussed at the end of the chapter, synovial sarcoma should be considered in the differential diagnosis of a pleural tumor with a biphasic or monophasic spindle cell appearance.40,41
Figure 79-4 Electron micrograph of a human mesothelioma cell showing abundant microvilli arising from the cell surface and prominent desmosomes (×10,500; inset, ×30,000). (Used with permission of Dr. Giuseppe G. Pietra, Department of Pathology and Laboratory Medicine, University of Pennsylvania Medical Center, Philadelphia.)
 MOLECULAR PROFILING
MOLECULAR PROFILING
As compared with routine histologic evaluation and classification, the examination of multiple expressed genes and/or proteins within individual tumors may be more informative for making diagnoses, estimating prognosis, and response to therapy. The development of microarray methodology, which permits the expression of thousands of genes to be assayed simultaneously, represents a powerful technique to read the “molecular signature” of an individual patient’s tumor, a process termed gene expression profiling. Gene expression profiling studies have been used to identify genes with potential pathogenic significance, such as aurora kinases or key inhibitors of apoptosis proteins. Profiles have also been identified that help to reliably differentiate different subtypes of mesothelioma. By using gene expression ratios, it is possible to reliably distinguish between epithelioid mesothelioma and lung adenocarcinoma or ovarian carcinomas from peritoneal mesotheliomas.42,43 An area of active investigation is the use of expression profiles as a means of predicting outcome and clustering groups of patients with pleural mesothelioma into those with good risk (i.e., more likely to be cured using aggressive therapy) and poor risk disease (i.e., with a low cure rate despite aggressive therapy). Some groups have found this approach to be highly predictive, whereas others suggest the accuracy has been overestimated.44
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
Malignant pleural mesothelioma most commonly presents in the fifth to seventh decades of life. Most patients diagnosed with mesothelioma earlier in life have a history of childhood asbestos exposure. The most frequent presenting symptoms of pleural mesothelioma, which are caused by the presence of extensive intrathoracic disease, are nonpleuritic chest pain (60%–70% of patients), dyspnea (25%), and cough (20%). Some patients are asymptomatic at diagnosis, with unilateral pleural effusions found incidentally on routine chest radiographs. Mesothelioma is typically a unilateral disease—only 10% of patients with mesothelioma have bilateral involvement at presentation. In more advanced stages of disease, physical findings may include unilateral dullness to percussion and decreased air movement throughout the hemithorax, asymmetric chest wall expansion during respiration, palpable chest wall masses, and scoliosis toward the side of the malignancy.
 RADIOGRAPHIC PRESENTATION
RADIOGRAPHIC PRESENTATION
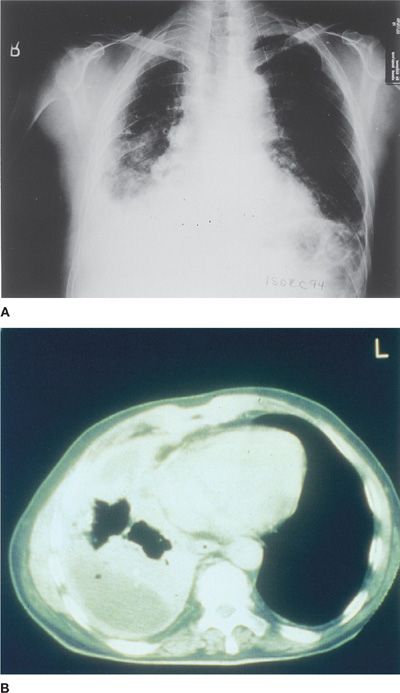
The most common initial radiographic manifestation of pleural mesothelioma is a large unilateral pleural effusion, often with contralateral mediastinal shift (Fig. 79-5A). Sixty percent of patients have right-sided lesions, putatively related to the gravitational predilection for inhaled asbestos fibers and dusts to travel directly to the right lower lobe airways. Occasionally, mesothelioma can present as unilateral, concentric, plaque-like, or nodular pleural thickening, but effusions may obscure the presence of the underlying pleural thickening. The tumor frequently extends into the fissures, which become thickened and irregular in contour. Only 20% of patients with pleural mesothelioma have radiographic signs of asbestosis (i.e., bibasilar interstitial fibrosis), although many have evidence of pleural plaques and/or calcifications. In later stages of disease, ipsilateral mediastinal shift is seen secondary to encasement of the lung by a thick rind of tumor and resultant significant unilateral loss of lung volume. Patients with advanced mesothelioma may also have other radiographic signs of volume loss: diaphragm elevation and intercostal space narrowing. They may also have mediastinal widening owing to direct tumor invasion or lymph node involvement, enlargement of the cardiac margins secondary to pericardial invasion with effusion, and evidence of rib destruction or soft tissue masses extending from the chest wall.45 Chest computed tomography (CT) is important in detecting invasion of chest wall, ribs, and mediastinal structures, as well as extension along the pleural surfaces and diaphragm (Fig. 79-5B). Furthermore, CT scans can distinguish between pleural thickening and effusion.46 Coronal magnetic resonance imaging (MRI) is helpful in discerning the extent of disease, particularly invasion of the chest wall, endothoracic fascial involvement, and extension of pleural mesothelioma through the diaphragm into the peritoneal cavity.47 In one study of 95 patients with pleural mesothelioma, MRI was directly compared with CT scan. The overall diagnostic accuracy for mediastinal nodal disease was approximately 50% for both modalities, but MRI outperformed CT for detection of diaphragmatic invasion (82% vs. 55% accuracy, respectively), and for detecting invasion of endothoracic fascia or chest wall (69% vs. 46%).48
Figure 79-5 A. Posteroanterior chest radiograph in a patient with malignant pleural mesothelioma demonstrating significant right-sided pleural effusion and diffuse pleural thickening associated with marked volume loss of the right hemithorax. No definite calcified pleural plaques are seen. B. Computed axial tomographic image from a patient with pleural mesothelioma, illustrating complete encasement of the ipsilateral lung with a thick rind of tumor, neoplastic invasion of the interlobar fissures, small residual pleural effusion, and marked unilateral volume loss.
 POSITRON EMISSION TOMOGRAPHY
POSITRON EMISSION TOMOGRAPHY
The role of positron emission tomography (PET) imaging, particularly PET/CT, in the care of patients with mesothelioma is multifold. It can be used in diagnosis and staging by evaluating the extent of pleural disease, establishing mediastinal lymph node involvement, evaluating tumor invasion into the lung and thoracic wall, and detecting extrathoracic metastases. It is becoming particularly useful to assess the treatment response to chemotherapy and radiotherapy and also plays an important role in the planning of radiation therapy.
The role of PET scans with 18-fluorodeoxyglucose (FDG) in staging and preoperative evaluation is evolving. In a small study of 28 patients with suspected pleural mesothelioma who underwent 18-FDG PET scanning followed by thoracoscopic or open surgical biopsy, PET was shown to be better than CT for differentiating malignant from benign pleural processes. Uptake of FDG was significantly higher in malignant lesions, and an overall sensitivity and specificity of 91% and 100% could be achieved with PET scanning for the detection of malignant as compared with benign disease. However, hypermetabolic lymph nodes were detected in 12 patients (of whom nine had a normal CT scan), and only five had histologically proven malignant nodal disease.49
In another small study, PET assessment demonstrated pleural lesions in 12 of 13 patients with malignant pleural disease (malignant pleural mesothelioma in ten patients, adenocarcinoma in two and liposarcoma in one), also revealing distant metastases in two patients. A patient with an epithelial mesothelioma had a false-negative result. In a study of 16 patients with pleural changes, PET correctly identified all 12 malignant cases; conversely, the four patients (4/4) who had no FDG uptake all had benign pleural disease (fibroma, tuberculous pleurisy, empyema, and pleural fibrosis).50
PET scan appears more sensitive than CT for finding extrathoracic disease, but has limited sensitivity for locoregional staging (i.e., determining potential resectability). In one retrospective study, 60 patients with malignant pleural mesothelioma were identified who had undergone PET scans preoperatively and the results of clinical staging were compared with surgical and pathologic results. FDG uptake was detected in 59, and the one false-negative case had disease limited to the parietal pleura (stage IA). The sensitivity of PET scans for determining the presence of T4 (unresectable) disease was only 19% (7 of 21 patients). Among the 31 patients whose nodal status was assessed pathologically, only one of nine patients with N2 disease was correctly identified by PET scan, and the overall sensitivity for nodal disease was only 11%.51
PET scanning may help differentiate between benign and malignant pathology when patients present with a chest radiograph demonstrating pleural abnormalities. One study demonstrated that dual timepoint FDG PET scanning, which measures uptake of (18)F-FDG over time, could distinguish between benign and malignant pleural diseases among 55 suspected cases.52
One of the potential future uses of PET that needs to be further evaluated is its utilization in the screening of patients with a history of significant asbestos exposure. These patients may potentially harbor microscopic disease, not apparent on CT or MRI, which may be amenable to early aggressive therapy. Because of the limits of detection of current 18-FDG PET technology, the use of PET for screening for pleural mesothelioma must await the development of novel radiopharmaceuticals.
PET scans can also be used to predict survival and response to therapy. One study involving 177 patients with mesothelioma found that patients with tumors demonstrating intense FDG avidity (standardized uptake value >5) had a far worse prognosis. Another study showed that decreased radiopharmaceutical uptake on follow-up PET scans performed early after treatment may be an excellent predictor of overall clinical response.53 Furthermore, there is evidence supporting the use of PET scans after neoadjuvant chemotherapy to determine those who will benefit most from moving onto surgical resection.54
 LABORATORY STUDIES
LABORATORY STUDIES
Although there are no specific pleural fluid biomarkers for malignant mesothelioma, evaluation of pleural fluid chemistries may still be beneficial. Effusions associated with mesothelioma are strongly exudative, with elevated protein concentrations in the range of 4 to 5 g/dL and a lymphocytic predominance. Pleural fluid lactate dehydrogenase (LDH) concentrations often exceed those of patients with carcinomatous pleural effusions, with levels greater than 600 IU/L. In patients with advanced disease and extensive involvement of visceral and parietal pleura, pleural fluid pH and glucose are commonly low. In patients with mesothelioma, the presence of a low pleural fluid pH denotes both a poor overall prognosis and refractoriness to achieving successful palliative pleurodesis. In addition, the pleural effusion associated with mesothelioma is characteristically highly viscus, presumably because of elevated concentrations of hyaluronic acid.55 An increased pleural fluid hyaluronidase level is suggestive but not diagnostic of mesothelioma.56 The cytokine profile of pleural effusions related to mesothelioma is somewhat unique in that the tumor constitutively produces high concentrations of interleukin-6 (IL-6) and transforming growth factor-β (TGF-β), but relatively low levels of IL-1β and TNFα. These elevated intrapleural levels of IL-6 in patients with malignant mesothelioma are postulated to induce systemic manifestations such as fever, cachexia, and thrombocytosis.57,58
Pulmonary function testing typically demonstrates a restrictive pattern resulting from pleural effusions, tumor encasement of the lung, or chest wall involvement.
 MESOTHELIN AND OTHER NOVEL SERUM MARKERS
MESOTHELIN AND OTHER NOVEL SERUM MARKERS
There is increasing evidence supporting the clinical utility of a monoclonal antibody–based serum assay for a soluble form of the protein mesothelin (soluble mesothelin–related peptide [SMRP]). Mesothelin is a 40-kDa glycoprotein that is found in low levels on the cell surface of normal mesothelial cells (lining the pleura, peritoneum, pericardium, and tunica vaginalis), but is highly expressed on mesothelioma, pancreatic cancer, and ovarian cancer cells.59,60 Multiple studies have observed that SMRP can be elevated in serum and pleural fluid of patients with mesothelioma.61 Increased levels of SMRP were found in serum samples from 37 of 44 patients with mesothelioma (87%), compared with 3 of 160 patients with other cancers or inflammatory lung or pleural diseases (2%), and none of 28 controls without a past asbestos exposure.62 At the present time, SMRP levels play an adjunctive role in the diagnosis of patients with mesothelioma. A meta-analysis of 16 diagnostic studies found that the sensitivity ranged widely from 19% to 68%, depending on the specific criterion for positivity.61 SMRP has also been measured in pleural fluid. In a retrospective study of 52 patients with malignant mesothelioma, the assay had a sensitivity of 67% with a specificity of 98% in 84 patients with benign pleural effusions.63 It is intriguing to posit that SMRP may also play a role in screening of high-risk patients for incipient mesothelioma. In one report, 7 of 40 asbestos-exposed individuals had elevated levels and 4 of these 7 subsequently developed mesothelioma or lung cancer within 1 to 5 years.62 However, measurement of SMRP is indicated currently only for monitoring patients in whom the diagnosis has already been established.
Osteopontin, a glycoprotein that mediates cell–matrix interactions and is overexpressed in several types of cancer, was higher in patients with malignant mesothelioma than in patients with asbestos-related nonmalignant pleural disease or no prior asbestos exposure in a study of 190 patients.64
Fibulin-3 is an extracellular glycoprotein that is encoded by the epidermal growth factor–containing fibulin-like extracellular matrix protein 1 (EFEMP1) gene. Initial studies found that elevated levels of fibulin-3 in the plasma had high sensitivity and specificity (97% and 96%, respectively) in distinguishing patients with pleural mesothelioma from those with a history of asbestos exposure but without mesothelioma and from those with other malignancies or benign causes of pleural effusion. Levels of fibulin-3 decreased in patient with mesothelioma who underwent surgical resection. Additional studies will be required to determine its role as a biomarker for early diagnosis and monitoring of patients who have undergone therapy.65
 DIAGNOSIS
DIAGNOSIS
The differential diagnosis of malignant pleural mesothelioma includes both benign and malignant processes. Inflammatory reactions such as chronic, organized empyema can mimic the dense pleural thickening and large, viscous pleural effusions characteristic of mesothelioma. As discussed, epithelial mesotheliomas can be extremely difficult to distinguish grossly and histologically from metastatic adenocarcinoma to the pleura from any number of primary sources, including lung, breast, stomach, kidney, ovary, and prostate. Sarcomas such as fibrosarcoma can present in similar fashion and infiltrate-like sarcomatoid mesotheliomas. The mixed-cellular (biphasic) subtype of mesothelioma can bear a significant histologic resemblance to sarcomatoid carcinomas and synovial sarcoma.
Thoracentesis or closed pleural biopsy can often establish the diagnosis of pleural malignancy, but may not provide enough diagnostic material to determine the specific diagnosis of mesothelioma. Immunohistochemical markers and monoclonal antibodies may aid in differentiating mesothelioma from other carcinomas on cytology specimens. In addition, certain cytopathologic features of cells obtained from pleural fluid have been found to correlate well with the presence of mesothelioma, including papillary aggregates, multinucleation with atypia, cell-to-cell apposition, nuclear pleomorphism, and macronucleoli. Gene expression ratios may also be increasingly helpful in this regard. Negative results from thoracentesis and/or pleural biopsy do not exclude the diagnosis of mesothelioma and therefore surgical biopsy, which has a higher diagnostic yield, should be pursued in patients with high clinical suspicion.
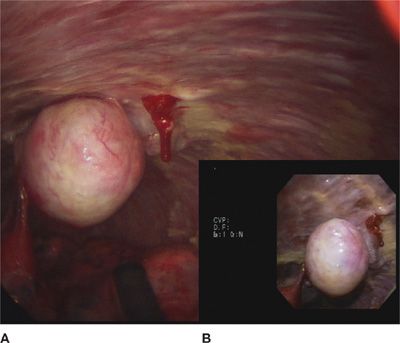
Surgical intervention, via video-assisted thoracoscopic surgery (VATS) or open thoracotomy, is often necessary to firmly establish the diagnosis. Boutin et al. from Marseille prospectively evaluated VATS for the diagnosis of malignant pleural mesothelioma in 188 consecutive patients from 1973 to 1990 and found that thoracoscopic biopsy was diagnostic in 98% of cases, compared with only 26% for thoracentesis alone, and 39% for fluid cytology and closed pleural biopsy. These thoracoscopic procedures were performed under local anesthesia in an endoscopy suite with minimal morbidity or complications.66 It is important to note that approximately 10% of patients who undergo a transthoracic diagnostic procedure for mesothelioma may seed the biopsy site with tumor cells, later developing chest wall recurrences. This complication can potentially be prevented by prophylactic radiation therapy to the surgical incision or thoracentesis sites (Fig. 79-6).
Figure 79-6 A. View of a mesothelioma tumor in the pleural cavity attached to the visceral pleura through surgical thoracoscope. B. View of the same tumor through a flexible medical pleuoroscope.
Concurrent bronchoscopy may be important in distinguishing between mesothelioma and metastatic adenocarcinoma of the lung, as endobronchial lesions are rarely seen in mesothelioma. In addition, both endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and mediastinoscopy play important roles in the diagnosis and staging of mesothelioma, as several studies have documented the significant negative prognostic implications of mediastinal nodal invasion in this disease. In comparative trials, EBUS-TBNA may actually have a higher sensitivity and specificity for determining mediastinal nodal involvement than mediastinoscopy.67,68
 STAGING
STAGING
The staging of malignant mesothelioma has proved to be more controversial than that of many other tumors. The most commonly used schema was devised by Butchart in 1976 (Table 79-1). Although useful, its ability to predict survival is weakened by lack of inclusion of lymph node involvement and chest wall invasion. For this reason, the Union Internationale Contre le Cancer (UICC) in 1990 first proposed a staging system based on the TNM (tumor/node/metastasis) standard used for many other tumors. In the TNM staging system, stages I and II disease have pleural involvement, potentially including diaphragmatic muscle or pulmonary parenchyma, but no evidence of lymph node involvement, distant metastases, or locally advanced, unresectable disease. Stage III mesothelioma includes those cases with regional lymph node involvement. Stage IV includes those with locally advanced and unresectable disease, contralateral lymph node involvement, supraclavicular lymph node involvement, and/or distant metastases.69 More recently, Rusch et al. from the International Mesothelioma Interest Group (IMIG) proposed an updated staging system based upon tumor descriptors, providing precise anatomic definitions of the local extent of the primary tumor. This staging system (Table 79-2) was designed to provide the framework for proper analysis of prospective clinical trials of new treatment modalities.70