Malignant Mesothelioma
Bruce W.S. Robinson
Paul Baas
Hedy Lee Kindler
Malignant mesothelioma (MM) is an aggressive malignant tumor of the pleura and other serosal surfaces, such as the p eritoneal and occasionally other serosal surfaces. It was c onsidered a rare disease until 50 years ago, but has increased dramatically in incidence since that time. This increase is caused by the widespread use of asbestos fibers in the postwar industrial period.
We will summarize the main features of the disease and provide an update of recent developments, focusing on new approaches to therapy for this otherwise treatment-resistant problem.
EPIDEMIOLOGY
In 1960, Wagner et al.1 reported an association between asbestos and both pleural and peritoneal MM in a South African case series. Since then, many reports supporting the relationship between occupational or environmental exposure to asbestos and the subsequent development of MM have been published.2,3
That initial study identified risks from both direct occupational exposure and brief or indirect exposure to asbestos.4 If asbestos exposure occurs at a young age, then the lifetime risk of development of MM is higher than in someone whose exposure occurs at a later age.
Around 80% of individuals with MM have an identifiable exposure to asbestos. Therefore, in around 20% of cases, there is no obvious exposure to asbestos and examination of the lung mineral fiber content shows that in many of these subjects there is a lower lung fiber burden than seen in subjects with asbestosis.5 MM may occur after brief and indirect exposure to asbestos. MM patients generally, though, have markedly increased lung fiber burdens when compared with a reference population.
Asbestos fiber dimensions and type play an important role in the development of MM, with longer and thinner asbestos fibers more likely to cause MM than shorter and wider fibers because they can penetrate the lungs (see discussion that follows). The critical fiber dimensions appear to be less than 0.25 mm in diameter and greater than 5 mm in length to produce MM, and, although the risk of developing MM from exposure to chrysotile fibers is lower than that from amphibole fibers, large amounts of chrysotile can cause MM, possibly because of contaminating tremolite fibers.6 A potential role for SV40 is also described (see discussion that follows).
PATHOGENESIS
Mesothelial Tissues Mesothelial tissues include all those that line the cavities that were derived from the embryonic mesodermal coelomic cavity. The tissue develops as a continuous epithelial layer, which covers the pleura, the pericardium, and the peritoneal cavity. A single layer of mesothelial cells rests on a basement membrane. Their rate of division is slow, but increases in response to inflammatory damage.7
Etiological Agents
Asbestos Asbestos types include serpentines, which are short and curved, such as chrysotile; and amphiboles, which are long and needlelike, such as crocidolite. Not all of the different forms have had widespread commercial use—in fact, 90% of industrial asbestos is chrysotile. The mining and use of asbestos is now in decline because of the related pulmonary conditions such as pleural plaques, diffuse pleural thickening, asbestos-related pleural effusions, lung cancer, and MM.8,9
Mesothelial cells have been shown to be 10 times more sensitive than bronchial epithelial cells to the direct cytotoxic effects of asbestos fibers.10 The fibers cause iron-catalyzed generation of reactive oxygen metabolites, which can have a direct toxic effect, causing DNA mutations and strand chromosomal breaks11,12 leading to cellular apoptosis.13 The end result is malignant transformation.
SV40 The double-stranded DNA virus SV40 has been suggested as a possible factor in the development of MM.14
Carbone et al.15 found SV40-like sequences in 60% of frozen MM specimens using polymerase chain reaction (PCR).16 SV40 is dependent on its host for the enzymes of replication except for the large T antigen (TAG). When the virus infects a cell, the TAG is transcribed from the viral genome. TAG binds to the specific SV40 origin of replication, pulling apart the DNA strand, allowing viral DNA synthesis. In this way, the virus is able to bypass the normal cellular controls on replication, and will even do so in quiescent cells. TAG binds to both p53 and the retinoblastoma protein (pRb), with inactivation of these cell cycle checkpoints.
Carbone et al.15 found SV40-like sequences in 60% of frozen MM specimens using polymerase chain reaction (PCR).16 SV40 is dependent on its host for the enzymes of replication except for the large T antigen (TAG). When the virus infects a cell, the TAG is transcribed from the viral genome. TAG binds to the specific SV40 origin of replication, pulling apart the DNA strand, allowing viral DNA synthesis. In this way, the virus is able to bypass the normal cellular controls on replication, and will even do so in quiescent cells. TAG binds to both p53 and the retinoblastoma protein (pRb), with inactivation of these cell cycle checkpoints.
It is presumed that SV40 was introduced into humans as a result of the Salk polio vaccines used in the 1950s, because up to 30% of vaccines used were contaminated by SV40 as a result of culturing the poliovirus in rhesus monkey kidney cells.
Several other studies have confirmed Carbone’s findings, with the proportion of cases ranging from 44% to 86% of MMs tested. Dissenting groups include that of Strickler et al.17 who examined MM tissue from 50 patients with two separate primer sets and did not detect any SV40 sequences. This group also undertook a retrospective cohort study comparing those people who were likely to have received contaminated polio virus against those who did not and found no increase in the incidence of several cancers, including mesothelioma.18
SV40 sequences present in MM tissue samples retain the ability to inactivate both p5319 and pRb,20 enabling a tumor to survive and progress. Clearly, SV40 is not essential for the development of MM, because many cases do not express TAG sequences. Further investigations and more accurate molecular and proteomic reagents are required to determine more clearly how SV40 fits into the pathogenesis of MM.
Other Agents Several other possible agents have been proposed to cause MM. These include thoracic radiotherapy, intrapleural thorium dioxide, and other silicates, including erionite and zeolite. The numbers of cases attributed to radiation exposure are very small. A genetic predisposition has also been suggested, but numbers are small and coexposure is difficult to exclude. There is no known association between smoking and MM.21
MOLECULAR LESIONS
A single mesothelial cell likely develops a genetic mutation that enables it to proliferate and overcome negative growth-stimulatory signals. A multistep accumulation of further mutations to cells o ccurs, producing the hallmarks of malignancy, namely autocrine growth, invasion, and the ability to metastasize.22 This whole process takes many years. These alterations include oncogene activation or mutation, loss of tumor suppressor genes, and autocrine or paracrine secretion of growth factors. In MM, considerable new information is available regarding candidate factors, but no clear single pathogenic pathway has been found.
Chromosomal Abnormalities Asbestos is known to induce chromosomal mutations. Cytogenic studies have shown many karyotypic changes,23 and a wide range of complex and heterogeneous chromosomal abnormalities have been described. Chromosomal gains have been found to be as frequent as losses, such as loss of 4, 22, 9p and 3p, and gain of 7, 5, and 20.24 The mean chromosomal number has also been shown to correlate with survival in patients with MM. Those patients with a normal chromosome number and no clonal abnormalities had the longest survival.25
There are some alterations that are of particular interest in terms of pathogenesis. Monosomy 22 correlates with mutations in the neurofibromatosis type 2 (NF2) gene.26 The loss of at least one locus in 1p (nearly all in 1p22) was found in 74% of examined specimens27; 42% to 62.5% of MM cases have been found to have loss of heterozygosity of one or more loci on chromosome 3p, the location of a gene for cellular senescence on chromosome 1 and a tumor suppressor gene located on chromosome 3. Polysomy of chromosome 7 is common and is a negative prognostic feature.28 The loci for the two potentially relevant growth regulators epidermal growth factor receptor (EGFR) and the platelet-derived growth factor A chain (PDGF-A) are both present on this chromosome. Deletions of 9p,29 the location of the gene for p16ink4 are also common, as are allelic losses in 6q in four discrete locations.
Oncogenes The oncogenes c-fos and c-jun have been implicated in animal models, with the levels of both c-fos and c-jun mRNA upregulated when rat pleural mesothelial cells are exposed to asbestos.30 Wild-type K-Ras was found in all 20 MM cell lines examined.31 c-Myc immunocytochemical expression is common,32 but c-myc is not amplified in murine MM cell lines.33
Tumor Suppressor Genes For a tumor to grow, it is necessary that normal cellular processes for inhibiting growth and for detection of damage be impaired. Tumor suppressor genes do this, so loss of a tumor suppressor gene enables an altered cell to continue through the cell cycle unchecked.34 Alterations in p53 have been found in 75% of murine MM cell lines35 but wild-type p53 was normally expressed in most human MM cell lines36 and primary tumors.37
The retinoblastoma protein pRb prevents progression of a damaged cell into S phase, but its level of expression in human MM cell lines has been shown to be normal.38
The product of the CDKN2 gene, p16 ink4, was found to be abnormally expressed in 12 of 12 primary MMs and 15 of 15 MM cell lines.39 As p16ink4 normally inhibits phosphorylation of pRb, its loss would permit progress through the cell cycle. Deletions of the portion of chromosome 9 containing CDKN2A, but not CDKN2B, were also found in MM cell lines.40 P16 has previously been found to be deleted in 85% of MM cell lines but only 22% of primary tumors.41
Seventy-two percent of primary MMs have also been found to have codeletions of p15 and p16.42 P16/CDKNA2 was homozygously deleted in 59 out of 80 human tumors.43 Patients with intact p16 had a significant survival advantage.44
The NF2 gene was found to be mutated in 41% of MM cell lines examined by Sekido et al.45 and 53% of cell lines examined by Bianchi et al.,46 and confirmed to be present in the primary tumor.
The Wilms’ tumor gene (WT1) is expressed in normal mesothelium. WT1 proteins control the transcription of genes such as those for PDGF-A, insulin-like growth factor (IGF)-II,47 transforming growth factor (TGF),48 and the IGF-I receptor (IGF-IR),49 which have been described as potential autocrine growth factors in MM. No inverse correlation was found between expression of WT1 and IGF-II or PDGF-A.50 A further study using mutational screening found no significant changes to WT1, and no correlation between WT1 immunostaining and EGFR or IGF-IR levels.51
GLOBAL TRANSCRIPTIONAL PROFILING IN MALIGNANT MESOTHELIOMA
Various global transcription profiling “microarray” strategies have been used in MM with various aims: to understand the genetics and biology of MM, to identify genes that may help diagnosis, for determining prognosis, and to identify potential targets for new therapies.
Studies using human MM patient samples have shown activation of pathways common to the development of many cancer types, including the IGF-1, p38 mitogen-activated protein kinase (MAPK), Wnt/β-catenin and integrin pathways.52,53
Diagnostic strategies for distinguishing MM from lung adenocarcinoma based on gene expression profiling of cytology samples have been described.54,55
Some studies have concentrated on finding gene “signatures” that can be used to predict prognostic indicators for MM.56,57,58,59 However, clinical prognostication based on gene expression profiling is not superior to clinical parameters such as age, epithelial histology, lymph node status, and tumor stage.60
IMMUNOBIOLOGY
There is some evidence in MM that specific immune responses are initiated against the tumor during the course of the disease.60 A lack of tumor-infiltrating lymphocytes (TILs), as with many other tumors, has been attributed either to a lack of tumor antigens or to the secretion of immunosuppressive cytokines.
Recent work has suggested that an immune response is generated in a significant proportion (28%) of MM patients 61 using patient sera and a panel of human MM cell lines as in Western blot analysis. The titre increased with the progression of the disease and the MM-reactive antibodies were of the immunoglobulin G (IgG) class, indicative of immunoglobulin class switching, and hence the involvement, of CD4 “help.” This may lead to identification of tumor-associated antigens (TAAs) and potential vaccination strategies.
This malignancy may be susceptible to immunotherapy. Intralesional therapy with granulocyte-macrophage colonystimulating factor (GM-CSF) induced a partial response with intense lymphocytic infiltration in biopsy samples.62 Gene therapy involving the use of gene encoding cytokines has provided some encouraging results in animal models, but limited success in patients.63
MMs have to secrete several cytokines known to modulate immune responses, including TGF-β and interleukin 6 (IL-6).64,65 The role of these molecules in MM has not been fully investigated in humans.
Human and murine lines all express class I molecules and thus can still be targets for the immune response.
ANIMAL MODELS
Spontaneous mesotheliomas have not been described in mice and occur very rarely in rats. Natural and synthetic fibers, chemicals, and metals, however, can induce pleural and peritoneal mesotheliomas in rodents.66 Inoculation of hamsters with SV40 virus causes pleural mesotheliomas.67 Mouse mesotheliomas are comparable to the human disease with respect to latency, growth patterns, and molecular lesions.68 These tumors have been used to study the immunobiology of MM69 and to evaluate chemotherapies, immunotherapies, and other therapies.70,71,72
Genetically modified transgenic mouse mesothelioma models have been developed. These models will be used for testing of drug therapies and study of the biology of the disease. Heterozygous p53 +/− knockout mice have a longer life span, and 76% of these mice had developed asbestos-induced mesothelioma, compared with 32% wild-type mice, at 44 weeks after asbestos exposure.73
Nf2 +/− knockout mice exposed to asbestos develop mesothelioma more rapidly and at a higher incidence than wild-type littermates.74,75 These tumors recapitulate the most common molecular features of human MM.76
A novel transgenic mouse model uses MexTAg, which directs SV40 Tag expression to the mesothelial compartment using the mesothelin promoter.77 When MexTAg mice are injected with asbestos, all of the animals develop MM and the disease occurs much more rapidly than in wild-type mice. This model is suitable to examine the efficacy of preventative and therapeutic drugs and also to investigate the molecular events occurring at the early stages of MM development.
Applications to Therapy Interventions that target factors such as TGF-β may be very effective from two aspects: first, by altering the growth cycle and, second, by permitting an immune response to be generated. When TGF-β was reduced by inhibiting translation of these proteins using antisense DNA technology, tumor growth was inhibited but not blocked completely—these effects were lost on cessation of treatment. Such approaches are worthy of further i nvestigation—possibly in combination with other treatments.
PATHOLOGY
There are several reasons for securing the diagnosis early in the course of the disease. Accurate classification is nowadays considered very important because of differences in the (natural) course of disease between MM and other tumors, and even between different subgroups of MM. The response to therapy can significantly influence both the response rate and survival time and will influence the results of single-arm phase II s tudies. In the last 2 decades, financial reimbursement programs have been initiated in many developed countries. Over and above a history of occupational asbestos exposure, this has also led to a greater demand for a definitive diagnosis.
In general, cytological examination of pleural effusion can accurately diagnose MM but cannot differentiate between mixed forms and epithelial type of MM. However, the accuracy of cytological diagnosis depends on the experience of the pathologist and the antibodies chosen.78 Fine-needle biopsies are recommended for the diagnosis of MM in some cases but are associated with low sensitivity (around 30%). Despite an increase in incidence of MM, the frequency is still relatively low. Thus, many local pathologists will have relatively little experience with this tumor, which often poses a diagnostic challenge, even to experienced pathologists. This is caused by the combination of highly variable histological features, often only low-grade nuclear changes and the need to identify invasion for definitive diagnosis. The diagnostic material presented to the pathologist is often inadequate, and the inexperienced pathologist may struggle to attempt a diagnosis when the safer option is to ask for better samples.
Such problems in diagnosis have resulted in the f oundation of so-called national and international mesothelioma panels. Their task is to optimize the diagnostics of pleural tumors, to give recommendations on further diagnostic requirements, to check reproducibility of tests that require stringent methodological procedures, and to validate newly reported immunohistochemical staining methods regarding sensitivity and specificity for use in routine diagnostic practice. Furthermore, these panels have been approved as official legal organs in financial reimbursement cases in many countries.
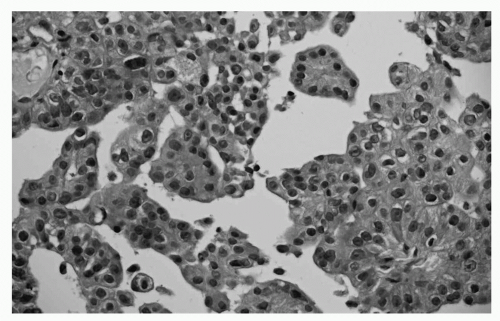 FIGURE 65.1 Reactive mesothelium. Superficial biopsy of the parietal pleura. There is no sign of infiltration, and a reactive mesothelial proliferation is the preferred diagnosis. (See color plate.) |
In patients presenting with pleural masses, large or multiple histological biopsies are preferred for diagnostic staining procedures.
MM can be differentiated into four subgroups including the epithelioid subtype (± 50% of cases), the biphasic subtype (20% to 25%), the sarcomatoid subtype (± 20%) and the desmoplastic subtype (1% to 5%). Older literature has clearly identified that diagnosis of the biphasic subtype is positively correlated with the number of biopsies taken during thoracoscopy or thoracotomy. In many pleural diseases, the mesothelial lining responds with a hyperplastic reaction, which might resemble MM (Fig. 65.1).79 One of the key features in diagnosing true cases of MM is, therefore, evidence of invasion of the underlying tissue layers (Fig. 65.2).80 This diagnosis cannot easily be made on small biopsies and cannot be assessed at all on cytology. Indeed, this is why many cytopathologists will not offer a definitive diagnosis; cytological diagnosis of MPM is presumptive at most centers.81
The collected material can be stained for many different markers. A combination of both “positive” and “negative” m esothelial markers should be used, taking into account the likely differential diagnosis in each case. To date, in Europe, it is advised that the diagnosis be made when the staining results i nclude at least two positive markers (nuclear markers such as anticalretinin and anti-WT1, the membrane marker anti-epithelial membrane antigen [EMA], and c ytoplasmic markers anti-CK5/6, antiD2-40 [podoplanin], antimesothelin) and two negative markers (anti-Ber-EP4, a membrane marker; antithyroid transcription factor-1 (TTF-1), a nuclear marker; monoclonal anti-carcinoembryonic antigen (CEA), anti-B72-3, anti-MOC-31 (antibody against epithelial glycoprotein 2), anti-estrogen receptor/- progesterone receptor (ER/PR), anti-EMA-cytoplasmic staining, selections based on likely differential diagnosis) (level of evidence 1A).82 The positive mesothelial markers
stain best in epithelioid tumor and are much less reliable in sarcomatoid tumor. The positive markers support the mesothelial nature of the cells but do NOT indicate their malignancy. The differential diagnosis of MM 83 consists in general of:
stain best in epithelioid tumor and are much less reliable in sarcomatoid tumor. The positive markers support the mesothelial nature of the cells but do NOT indicate their malignancy. The differential diagnosis of MM 83 consists in general of:
Primary adenocarcinomas of the lung involving the pleura
Metastatic disease from extrathoracic sites
Diffuse pleural sarcomas
epithelioid hemangioendothelioma
synovial sarcoma
leiomyosarcoma
Desmoplastic small round-cell tumor
Ewing sarcoma
Solitary fibrous tumors
Pleural thymoma
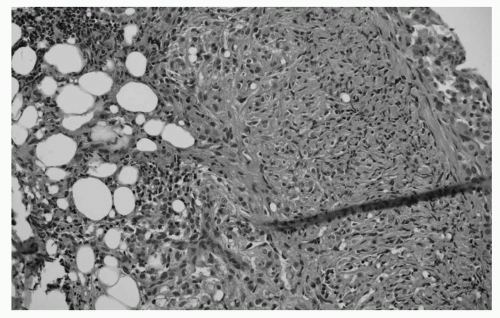 FIGURE 65.2 Deep biopsy showing invasion indicating MM. Deep biopsy from the parietal pleura in the same patient. Tumor cells show infiltration of the muscular layer and fat. The diagnosis of mesothelioma can now be confirmed. (See color plate.) |
CLINICAL PRESENTATION AND COURSE
Patients with pleural MM classically present with chest pain, dyspnea, cough, weight loss, fatigue, and sometimes fever or night sweats. Symptoms can persist for months or longer prior to diagnosis. Peritoneal MM patients present with increasing abdominal girth, abdominal pain or discomfort, constipation, anorexia, and occasionally an umbilical hernia. The most common presentation for a pericardial MM is death, because most are diagnosed postmortem. Other symptoms of a pericardial MM include dyspnea, fever, night sweats, congestive heart failure, constructive pericarditis, pericardial effusion, pericardial tamponade, and myocardial infarction. Tunica vaginalis MMs usually present with a unilateral t esticular mass.
For the patient with pleural MM, the physical exam is often unrevealing except for dullness to percussion, reduced air entry on auscultation, or asymmetric chest excursion. The disease tends to remain localized to the hemithorax until late in its course. The most common sites of metastases are to the mediastinal and hilar lymph nodes, contralateral pleura, lung, and peritoneal cavity. Metastases to liver, bone, and brain, although rare, can occur. Extensive local progression usually results in death, either from respiratory or cardiac failure.
MM is a heterogenous disease with a variable clinical course dependent upon several key prognostic factors. Using multivariate analysis, the Cancer and Leukemia Group B (CALGB) identified pleural involvement, high levels of lactate dehydrogenase, poor performance status, chest pain, thrombocytosis, nonepithelial histology, and age older than 75 years as poor prognostic factors. The CALGB defined six distinct prognostic groups, whose median survival ranged from 1.4 to 13.9 months.84 The prognostic scoring index developed by the European Organization for Research and Treatment of Cancer (EORTC) determined that poor performance status, probable diagnosis of MM, leukocytosis, male gender, and sarcomatoid subtype are indicators of poor prognosis. The EORTC classified patients into good-or poorprognostic groups; good-prognosis patients had a 1-year survival of 40%, compared with only 12% for patients in the poor-prognosis group.85 The EORTC prognostic score has been independently v alidated.86 In addition, measures of health-related quality of life (HRQOL), specifically pain and loss of appetite, may be independent prognostic factors in patients with advanced disease.87
IMAGING
The clinical manifestations of MM are usually nonspecific and insidious, but when complaints persist, a chest x-ray is performed to determine the additional diagnostic steps. Besides the presence of a unilateral pleural mass, chest x-rays can detect effusions and pleural plaques with/without calcifications. The presence of calcified plaques on the diaphragm indicates a probable exposure to asbestos in the past. Shrinkage of the afflicted hemithorax is compatible with more advanced cases of MM and can explain complaints of severe pain.
CT Scanning CT scanning is considered to be the most important method of radiological evaluation. It is not only useful to determine the extent of the disease but can also narrow the differential diagnosis. Signs of liver and adrenal metastases are unusual in MM and are only seen in very advanced cases. The following are other signs that point to the direction of MM:
soft tissue masses encasing the diaphragm, including the absence of a fat plane between the inferior surface of the diaphragm and adjacent abdominal organs
pleural tumor which may extend into the subcutaneous tissues at the site of a previous biopsy or thoracoscopy after weeks to months
the presence of pericardial effusion or nodular pericardial thickening
the possible involvement of mediastinal lymph nodes
shrinkage of the involved site
The diagnosis cannot be made based on the radiological appearance, nor can it be used alone for reliable staging. CT scanning will show the location and size of pleural and interlobular masses, allowing these lesions to be used as target lesions.
CT scanning is currently the method of choice for evaluation of response measurement in MM treatment and is used for follow-up after (combined modality) therapy. The accuracy of the CT scan to identify tumor-positive mediastinal lymph nodes is limited. Schouwink et al.88 performed a prospective study where preoperative CT scanning was compared with cervical mediastinoscopy and found a diagnostic accuracy of 67% for CT scanning and 93% for mediastinoscopy.
MRI Scanning MRI scanning has limited value in the diagnosis and staging of MM. It can be used when transdiaphragmal growth or involvement of major vessels or nerve plexus is suspected. Heelan et al.89 compared the accuracy of CT with MRI scanning for staging purposes. CT scan accuracy was identical to MRI for the detection of lymph nodes but superior in determining chest wall invasion.
PET Scanning Recently, the value of SUV (standard uptake values) in PET scanning has been identified as a new factor for both staging and prognosis purposes and validation studies are underway. Flores et al.90 investigated the sensitivity of PET scanning in 63 patients with different histology types of MM. He found that PET scanning was positive in all cases, showed a high false-positive rate for detection of mediastinal lymph nodes but identified an occasional, unsuspected, distant metastasis.
Erasmus et al.91 correlated the staging of PET/CT scanning in 24 patients. In seven cases, there was an understaging and in two cases, over staging of the T stage. For the N status, 35% were understaged and 29% overstaged. Flores et al.90 correlated the SUV value with survival in 137 patients. He observed an inverse relationship between higher SUV values and survival when a cutoff value of 10 was used.
PET scanning as an evaluation tool was tested by Ceresoli et al.92 in a group of 20 patients who received two courses of chemotherapy. Responders were defined as having at least a 25% reduction in SUV. In the group of r esponders (8) the median overall survival was 14 months, whereas in the nonresponding group (12), this was 7 months. The use of PET scanning is considered unreliable when patients have had a (chemical) pleurodesis. The resulting reactive pleuritis will lead to a highglucose uptake and leads to false-positive PET scan results. This can be positive up to 3 months after the p rocedure.
It is clear that PET/CT scanning contributes to the diagnosis and response evaluation of MM, but validation studies still have to be performed.
Biomarkers Measurement of tumor markers in effusions may provide a complementary tool to aid in effusion diagnosis. Although differential levels of CEA, cancer antigen (CA) 15.3, CA72.4, CA19.9, CA549, neuron-specific enolase, or cytokine fragment 19 (CYFRA 21-1) differentiate malignant from benign effusions,93,94 there is less data available for the differential diagnosis of MM from other cancers. Elevated CA15-3 levels have been reported in MM94,95,96 and in one study as being able to differentiate between MM and bronchial cancer.96 Higher levels of hyaluronic acid have been reported in effusions from MM patients compared with those with other malignant disease; however, the difference was too small for diagnostic purposes.97 Mesothelin levels in effusions above 20 nM are highly suggestive of malignancy, particularly of MM; at this cutoff value, the assay had a sensitivity of 77% for nonsarcomatoid MM, and a specificity of 98% relative to nonmalignant effusions and 86% relative to non-MM malignancies (Fig. 65.3).98 Mesothelin levels in the blood have been shown to be useful in diagnosis plus monitoring disease progress/regression.98
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


