Fig. 23.1
Schematic diagram of the lymphatic system demonstrating the superficial and deep lymphatics and lymph nodes
The lymphatic vasculature is composed of a hierarchal network of initial and collecting lymphatic vessels that exhibit molecular, cellular, and functional differences. Initial lymphatic vessels consist of a single layer of endothelial cells with end-to-end or overlapping junctions [1, 2, 6]. The basement membrane is scant or absent around the initial lymphatic vessels, which are connected to the extracellular matrix by fibrillin-containing anchoring filaments. These anchoring filaments may modulate the uptake of interstitial fluid through molecular signaling in addition to pulling apart endothelial cells. The initial lymphatic vessels drain into the collecting lymphatics (Fig. 23.2) [1, 6].
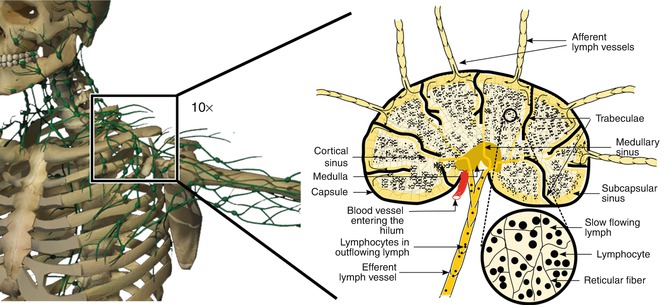

Fig. 23.2
Schematic diagram of the lymphatic vessels. Note the initial lymphatic vessels that are represented as solid green, smaller-diameter vessels that drain into the larger-diameter collecting lymphatic vessels. Note the smooth muscle cells around the collecting lymphatics and the presence of valves. Segments of collecting lymphatic vessels located between two valves are known as “lymphangions”
Collecting lymphatic vessels are composed of endothelial cells that are surrounded by a well-defined basement membrane. Intraluminal bicuspid valves are present within the collecting lymphatic vessels. Valves partition collecting lymphatic vessels into discrete contractile segments, termed “lymphangions,” which are surrounded by the smooth muscle and contract to actively transport lymphatic fluid through lymph nodes and throughout the lymphatic system (Fig. 23.2) [1, 2, 6].
Since the lymphatics lack a central pump, lymphatic fluid is propelled through the lymphatics through the concerted effects of respiratory motions, skeletal muscle contractions, and the autocontractility of the mural smooth muscle of the vasculature itself. In the skeletal muscle, lymphatics are usually paired with arterioles, so that arterial pulsations can also contribute to the periodic expansion and compression of the initial lymphatic vessels to enhance fluid uptake [1–3, 7].
23.3 Pathophysiology
Lymphedema is an imbalance between lymphatic fluid formation and lymphatic fluid absorption, representing a high-output or low-output failure of the lymphatic system or a combination of both. The increase of interstitial fluid leads to a cascade of remodeling that leads to permanent changes in the tissues of the affected limb [1–3, 7].
High-output failure (also known as dynamic insufficiency) occurs when excessive lymphatic fluid formation exceeds the transport capacity of the intact lymphatic system. Increases in lymphatic fluid production may arise when Starling forces shift net pressure to favor the flow of fluid into the interstitium. Increases in venous pressure result in increased hydrostatic pressure within the venules, and capillaries increase the driving force for ultrafiltration [1–3]. The loss of oncotic pressure, as seen in hypoproteinemic states such as malnutrition, has a similar effect. Elevated venous pressure occurs in patients with right heart failure, deep vein thrombosis, and venous insufficiency. Local inflammation increases capillary permeability, accelerating the loss of fluid and plasma proteins into the interstitium [1–3].
In contrast, low-output failure (also known as mechanical insufficiency) occurs when there is injury or impairment of the lymphatic system due to paralysis, obstruction, or inadequacy of the lymphatics (e.g., lymphedema from filarial lymphatic obstruction or congenital hypoplasia) [1–3]. As lymphatic obstruction progresses, tortuosity, dilatation, and pooling of lymphatic fluid give way to massive ectasia, valvular destruction, retrograde lymph flow, and lymph coagulation. Intrinsic truncal contractions fail; intraluminal valves give way, and hydrostatic pressure increases in the superficial valveless lymphatic watersheds. Chronic inflammation results in mast cell infiltration, disruption of the interstitial elastin fiber network, intense lymphangiogenesis and hemangiogenesis, fibrosis, progressive fat deposits, and skin thickening [1–3].
Dermal edema is the hallmark of lymphedema and represents the earliest clinical manifestation of lymphatic impairment [1, 2]. The presence of dilated lymphatic vessels may also be evident. With prolonged lymphatic impairment, tissue changes include fibroplasia, hyperkeratosis, and increases in stromal cells. In addition, elastic tissue fragmentation, clumping, and loss of mature elastic fibers also occur. Abundant subcutaneous fat becomes a predominant component of the swelling seen in the affected limb [3, 7].
The inflammatory cells present in the edematous tissue contribute to the ongoing fibrosis. It is believed that the inflammatory cells fail to migrate to the lymph nodes due to impaired lymphatic transport and dysfunctional lymphangiogenesis, leading to worsening edema and further inflammation. This ultimately results in impaired immune trafficking and decreased clearance of pathogens [1, 2].
Lymphedema is a progressive and usually painless swelling of the limbs or genitals that is the result of decreased transport capacity of the lymphatic system. Lymphedema can be primary or secondary. Primary lymphedema is due to a defect in the lymph conducting pathways. Secondary lymphedema is due to an acquired cause (such as filariasis, previous surgery, radiation therapy, malignancy, infection, and inflammation) that results in injury and impairment of the lymphatic system (Fig. 23.3) [2–4, 7, 8].
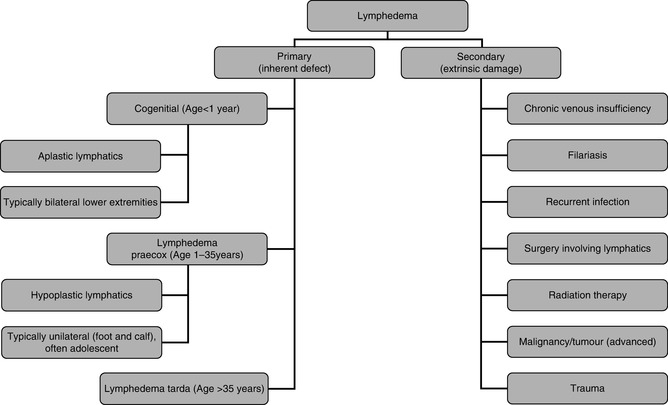

Fig. 23.3
Primary and secondary lymphedema. Lymphedema is the result of decreased transport capacity of the lymphatic system. Lymphedema can be primary or secondary. Primary lymphedema is due to a defect in the lymph conducting pathways. Secondary lymphedema is due to an acquired cause
23.4 Stages of Lymphedema
Regardless of the etiology, lymphedema is clinically staged by the extent of visible tissue degradation (Table 23.1) [9–12]. In the early stages of lymphedema (stage I), the associated limb swelling resembles other types of edema such as that seen with congestive heart failure, renal insufficiency, and venous disease. At this stage, the swelling is completely relieved with elevation and/or overnight rest. The tissues are soft and usually pitting with no evidence of fibrosis. As the condition progresses (stage II), the edema is no longer relieved with elevation or rest, and skin changes begin to appear such as induration of the skin and progressive hardening. The edema also becomes nonpitting. In the late, chronic stage of lymphedema (stage III), the edema is severe, and the skin is fibrotic with numerous skin changes that include hyperkeratosis, warty projections, cobblestoning, and lichenification (Fig. 23.4). Stage III lymphedema is also known as “elephantiasis” because the affected limb begins to resemble the leg of an elephant [9, 10, 13].
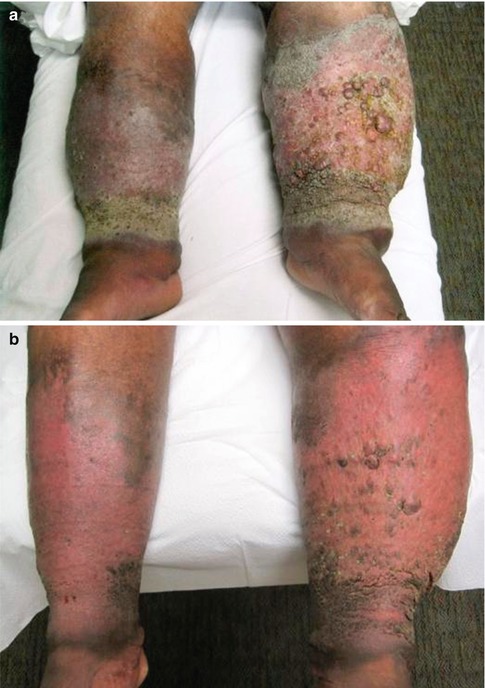
Table 23.1
Stages of lymphedema
Latency | Risk for lymphedema present. No clinical change evident |
Stage I | Pitting, reduces overnight with simple measures (elevation). No fibrosis |
Stage II | No longer pitting, no full reduction with elevation, evident fibrosis |
Stage III | Nonreversible, hardened fibrosis and sclerosis of cutaneous and subcutaneous tissues |

Fig. 23.4
Bilateral lower extremity lymphedema in a 56-year-old man before and after complex decongestive therapy. (a) Before treatment. Note the significant limb swelling and chronic skin changes (lichenification, warty projections, and cobblestone appearance) associated with lymphedema. (b) After treatment. Note the significant improvement in limb swelling and chronic skin changes
23.5 Primary Lymphedema
In patients with primary lymphedema, the cause of decreased lymphatic transport can be an intrinsic “defect” or a malfunction of the lymph conducting elements, which is believed to be a genetically determined abnormality of lymph drainage [2]. The majority of lymphedemas classified as primary lymphedema have inborn abnormalities of the lymphatic system that manifest mostly with irregular or abnormal structural development caused by abnormal (mutant) genes [2, 11]. These abnormalities result in lymphatic hypoplasia, aplasia, numerical hyperplasia, or dilation (lymphangiectasia) with valvular incompetence [2, 11].
Primary lymphedemas have been classified into three groups, depending on the age of onset: congenital (before age 2), praecox (onset between ages 2 and 35), and tarda (after age 35). Lymphedema praecox is the most common form of primary lymphedema with a female to male ratio of 10:1. It is usually unilateral and often limited to the foot and calf in most patients (Fig. 23.3) [2, 8, 11].
23.6 Secondary Lymphedema
Secondary lymphedema is far more common than primary lymphedema and represents 90 % of cases of lymphedema. The most common causes of lower extremity lymphedema are tumor (e.g., lymphoma, prostate cancer, ovarian cancer), surgery involving the lymphatics, radiation therapy, obesity, trauma, and infection. Worldwide, infection with the parasitic nematode Wuchereria bancrofti (also known as filariasis) is the most common cause of lymphedema (Fig. 23.3) [2, 4, 8].
23.7 Diagnosis
23.7.1 Clinical Evaluation
Evaluation of patients with lymphedema must include a detailed, careful history and thorough physical examination [3, 4, 7, 11, 12]. The history should include age at onset, travel to tropical countries, and history of all causes that could result in secondary lymphedema such as surgery, malignancy, venous insufficiency, trauma, and cellulitis. A history of temporary edema of the affected limb or other areas must be noted, and a detailed family history of limb swelling should also be recorded.
Signs and symptoms of lymphedema should be documented. These include nonpitting edema, skin changes such as “peau d’orange,” pinkish-red skin discoloration, hyperkeratosis, dermatitis, eczema, ulceration, varicosity, lymph vesicles, warty projections, drainage of fluid (clear or milky), or yellow discoloration or other abnormalities of the nails (Fig. 23.4). The presence of Stemmer’s sign (inability to pinch a fold of skin at the base of the second toe) or puffiness of the forefoot (buffalo hump) should be noted (Fig. 23.5) [3, 7, 11, 12]. The presence of venous, arteriovenous, or capillary malformations and any limb length discrepancy should be recorded. Finally, any complications such as cellulitis, lymphangitis, malnutrition, and immunodeficiency or, rarely, suspicion for malignancies (lymphangiosarcoma) must be documented [3, 7, 11].
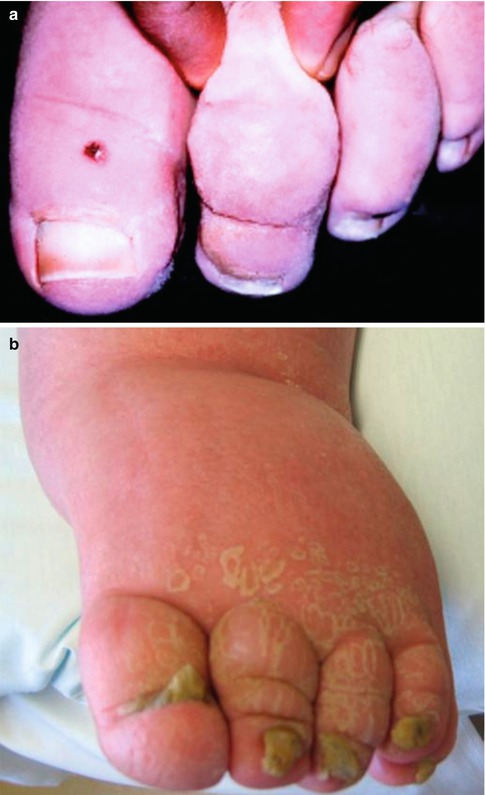

Fig. 23.5
Clinical signs of lymphedema. (a) Positive Stemmer’s sign (a failure by the examiner to pick up or pinch a fold of skin at the base of the second toe). (b) Buffalo hump on the dorsum of the left foot in a patient with lymphedema
23.7.2 Noninvasive Radiologic Studies
Plain film X-rays will identify limb length discrepancies, bone abnormalities, or phleboliths in patients with combined lymphatic malformations and venous malformations [7, 11].
23.7.3 Minimally Invasive Radiologic Studies
Radionuclide Lymphoscintigraphy
This study is performed with a subcutaneous injection of 99mTc-labeled human serum albumin (HAS) or 99mTc-labeled sulfur colloid (SC) into the first and second web space of the toes (fingers), followed by radionuclide scanning at various time intervals [7, 11]. It is the test of choice to confirm or exclude lymphedema as the cause of chronic limb swelling. Removal of the colloid from the injection site; appearance time of activity at the knee, groins, or axilla; absence or presence of major lymphatic collectors; number and size of vessels and nodes; the presence of collaterals and reflux; and symmetric activity with the opposite side are recorded and used for interpretation.
An appropriate combination of non- to minimally invasive tests normally should provide all the information necessary to insure an adequate diagnosis and lead to the correct multidisciplinary, specifically targeted and sequenced treatment strategy. The tests and the information they provide are indicated here [11].
Basic/essential tests:
Radionuclide lymphoscintigraphy
MRI with/without contrast for the differential diagnosis
CT scan to exclude underlying pathology
Duplex ultrasonography
Optional tests:
Whole body blood pool scintigraphy (WBBPS)
Magnetic resonance (MR) and/or ultrasound lymphography
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


