Lymphatic Drainage of the Esophagus
Linda W. Martin
Mark J. Krasna
The lymphatic anatomy of the esophagus is relevant to thoracic surgeons apropos of staging, treatment planning, surgical approach, and technical aspects of esophagectomy for esophageal cancer. This chapter highlights the intra- and extraesophageal lymphatic anatomy, abdominal and thoracic lymph node map specific to the esophagus, clinical implications, and new horizons in esophageal cancer staging and treatment in relation to lymphatic spread.
Anatomy
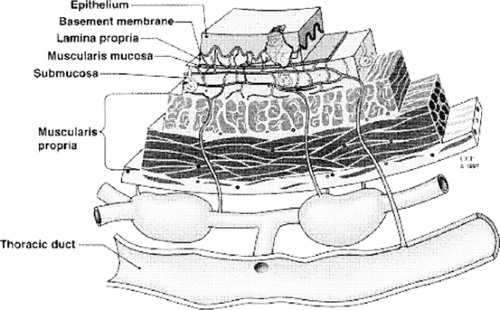
In 1903, Sakata published his study on the lymphatics of the esophagus based upon anatomic findings in 15 fetuses.31 In this seminal work, Sakata31 noted that the lymphatic drainage of the esophagus is mainly longitudinal and intramural rather than segmental. There is a dense submucosal lymphatic plexus, while the lymphatic network in the muscular layer and adventitia is less developed. The longitudinal submucosal network is continuous with the lymphatics of the pharynx and the stomach. Collecting trunks originating from the submucosal network intermittently pierce the muscularis propria to drain into regional lymph nodes (Fig. 130-1). They are able to penetrate the esophageal wall through transverse, ascending, or descending pathways of variable length. The collecting trunks may also connect directly into the thoracic duct in up to 40% of patients21,27,28 (Fig. 130-2). In another 10% of patients, lymphatic channels connect to the thoracic duct via a single paraesophageal (level 8) node.27
Theoretically, lymph within the submucosal plexus flows cranially from locations above the carina and toward lower mediastinal and infradiaphragmatic lymph nodes from the lower esophagus.16 However, this physiology is difficult to confirm and very well may be altered by lymphatic obstruction from inflammation or tumor infiltration. Thus the true direction of flow in disease states is difficult to estimate.
Lymph Node Anatomy and Mapping
The use of a precise numerical lymph node map has been critical to the design and conduct of clinical trials in lung cancer and has facilitated accuracy in comparing results among different investigators. For the same reasons, standardized use of lymph node mapping systems in esophageal cancer would be beneficial, but unfortunately its application has been suboptimal in reporting outcomes for this disease. The most commonly used maps are the Japanese system,8 which originated from a gastric cancer nodal mapping system, and the system first described by Casson and coworkers3 (Fig. 130-3), based on the lymph node map for lung cancer. The latter has been adopted by the American Joint Committee on Cancer (AJCC)1 for the staging of esophageal cancer; thus the following discussion focuses on this system.
This regional node map utilizes the mediastinal stations originally described by Naruke and coworkers22 for lung cancer. Modifications specific to esophageal cancer staging are 3P (posterior), for upper paraesophageal lymph nodes above the tracheal bifurcation, and station 8, which is divided into 8M (middle) and 8L (lower) paraesophageal lymph nodes, with the addition of stations 15 through 20. Station 15 is defined as the nodes posterior to the crura; station 16 is nodal tissue immediately adjacent to the esophagogastric junction; level 17 is along the course of the left gastric artery; common hepatic artery nodes are labeled level 18; nodes adjacent to the splenic artery are level 19; nodes located at the base of the celiac artery are level 20. Levels 8L (lower) and 16 are separated by the phrenoesophageal ligament.
The right recurrent laryngeal nerve (RRLN) node is a station that is not included in either of the two mapping systems, although it has recently received more attention in the literature. Its utility has been evaluated primarily in squamous cell esophageal cancer. Several Japanese studies20,35,37 report its potential use as a “sentinel” node for cervical node involvement in the selection of patients in need of three-field nodal dissection who have middle- and upper-third thoracic squamous tumors. It is located along the right recurrent laryngeal nerve, behind the right subclavian artery and between the trachea and the esophagus at the supreme portion of the thorax; most commonly it is accessed during right thoracotomy. The Japanese literature suggests that when the RRLN is positive, bilateral neck explorations should be performed for complete cervical nodal clearance, albeit with significant morbidity (38.4% vocal cord paralysis, 5.8% asphyxia, with one patient death35). This extensive lymphadenectomy thus far has not been adopted into standard
practice in western countries; recent data suggests that perhaps greater attention should be paid to cervical node disease irrespective of the location of the primary tumor.15
practice in western countries; recent data suggests that perhaps greater attention should be paid to cervical node disease irrespective of the location of the primary tumor.15
Clinical Implications: Patterns of Metastatic Spread in Esophageal Cancer
The anatomic descriptions outlined above can be applied to surgical practice in several ways. The incidence of metastases to lymph nodes in carcinoma of the esophagus ranges between 45% and 70% in different series.7,15,26,32 Factors such as depth of tumor invasion, tumor location within the esophagus, and histology all affect the probability of nodal metastases. Application of the anatomy and physiology described above provides clues to the surgeon as to when and where to look for nodal spread. Furthermore, the possibility of skip metastases can be explained by the described patterns of lymph drainage from the esophagus. It is important to keep in mind that under disease conditions, lymph channels may be altered and thus patterns of metastatic spread may not follow the same pathways as lymph flow under normal circumstances.
T Status
The importance of the pervasive submucosal network can be appreciated in terms of depth of tumor invasion and its correlation with lymph nodal metastases. The more extensive the tumor invasion, the greater the probability of nodal involvement. Depth of tumor invasion, or T status, was identified by Rice and colleagues26 as a significant predictor of regional lymph node metastasis (N1 disease). Compared with T1 patients, a T2 patient was 6 times more likely to have regional lymph node metastasis; a T3 patient, 23 times more likely; and a T4 patient, 35 times more likely (Table 130-1). Other authors19,23 have also found that the more deeply the tumor invades the esophageal wall, the higher the risk of metastatic lymph node spread.
The introduction of techniques for localized treatment of superficial esophageal cancer—such as endoscopic mucosal resection (EMR),17 radiofrequency ablation,36 photodynamic therapy (PDT),10 among others—has prompted practitioners to query the likelihood of nodal metastases based on subcategories of T status. The standard designations of T1 to T4 do not take into account the difference between mucosal and submucosal invasion within the T1 category. The Japanese have developed a classification system that designates three levels of mucosal and three levels of submucosal invasion.5 It is unclear whether this system is applicable to adenocarcinoma. However, a subdivision of T1 into T1a (tumor limited to the mucosa) and T1b (tumor invasion into the submucosa) has potentially useful prognostic information in either histologic type. Stein and colleagues34 reported the prevalence of nodal metastases after esophagectomy for early-stage tumors. For squamous tumors, 2 of 26 (7.7%) T1a lesions had nodal metastases, and 39 of 107 (36.4%) T1b neoplasms had positive nodes. For adenocarcinoma, 0 of 70 had nodal involvement with T1a status and 18 of 87 (21%) with T1b. Maish and colleagues18 have used EMR as a staging technique to pathologically confirm the T status prior to esophageal resection in order to determine the extent of nodal dissection required. Their results in 7 patients indicate that EMR accurately determined the depth of tumor invasion and led to complete removal of the tumor in all but 1 patient. Two patients had nodal dissections based on submucosal involvement on EMR.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree