Lung Volume Reduction
Anastasios Polimenakis
Keith S. Naunheim
History
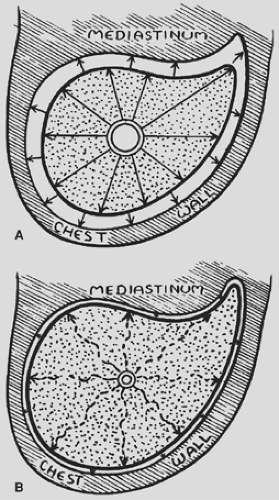
It was Otto Brantigan6 in the 1950s who first had the idea that localized lung resection might benefit patients with diffuse end-stage emphysema. It was his hypothesis that the inflammatory process of emphysema leads to destruction of lung tissue, with a resultant loss of parenchymal elasticity. This process resulted in a loss of the “tethering” effect that serves to hold open the walls of bronchioles throughout much of inspiration and expiration (Fig. 88-1). When the lung was destroyed, it led to a loss of this tethering, early closure of the bronchioles and hyperinflation. It was his supposition that by removing the most diseased portions of lung, one could restore the tethering effect thus maintaining patency of the bronchioles and improve airflow. Of the initial 33 patients on whom he originally reported, 6 died postoperatively, representing an operative mortality of 18%. Although Brantigan noted that there was subjective improvement in survivors, there were little or no objective data to support his statement and the operation fell into disuse.
Several decades later, this operative approach was taken up by Delarue14 and by Dahan10 in an attempt to improve function in patients with end-stage emphysema. Both investigators, like Brantigan, utilized a unilateral thoracotomy approach with excision of diseased lung. The two French investigators, however, based much of their decisions on the pulmonary hemodynamics and felt that it was perhaps the vascular physiology, with resultant hemodynamic changes, that caused improvement in dyspnea. However, the resultant improvement was not significant enough for this operation to become popular.
In 1991, Wakabayashi63 reported his results for the treatment of end-stage emphysema by unilateral parenchymal laser ablation via a thoracoscopic approach. Other investigators failed in their efforts to duplicate his results.21
It was not until 1994 that Cooper7 adapted Brantigan’s theories and performed his own series of lung volume reductions (LVRs); thereafter, the procedure became popular. Cooper utilized a median sternotomy approach with bilateral excision of diseased, emphysematous upper lobes. As a result, his patients demonstrated remarkable improvements in oxygenation, spirometry, quality of life, and exercise capacity. Perhaps most importantly, this occurred without a single operative mortality. Following Cooper’s report in 1995,7 there was a brisk and far-reaching dissemination of the LVR procedure for the treatment of patients with end-stage emphysema, and the modern era of LVR began. There were multiple technical variations, including the employment of a unilateral thoracoscopy approach,24,26,43 a bilateral thoracoscopy approach,4,29,33 and bilateral minithoracotomies14 in addition to the median sternotomy approach popularized by Cooper. The initial published reports were almost universally encouraging and documented rates of morbidity and mortality that were relatively favorable.
Rationale
On the surface, it would not seem rational to treat patients who demonstrate inadequate pulmonary reserve by removing significant amounts of lung tissue. However, in many patients, it does indeed improve their functional status. This has been explained through one of four pathophysiologic pathways.
Ventilation/Perfusion Mismatch
Patients with end-stage emphysema have distended airspaces; these may be inadequately ventilated yet still have some perfusion, resulting in the phenomenon of shunting. In addition, these distended airspaces may continue to expand and compress adjacent, well-perfused alveoli, which then become similarly dysfunctional. Theoretically, resection of such damaged lung would remove some component of the ventilation/perfusion mismatch and potentially allow reexpansion of adjacent alveoli, with a return to more normal function.
Airway Resistance
There is an inherent elasticity within lung tissue. After alveoli become maximally distended during inspiration, the elastic properties of the alveolar wall tend to produce a constrictive force driving air outward, similar to what occurs in a deflating balloon. In end-stage emphysema, the alveolar walls become inflamed and are less elastic, which subsequently leads to a loss of elastic recoil, with less outward driving force during exhalation. As first suggested by Brantigan,6 the intact lung parenchyma also has a supportive or outward tethering effect on the terminal bronchioles—a phenomenon that tends to hold the bronchioles open throughout the respiratory cycle and maintains their patency. Parenchymal destruction by emphysema leads to a reduction in the tethering effect.
The combination of the loss of the driving force of elastic recoil and the reduced tethering effect leads to closure of terminal
bronchioles earlier in the expiratory cycle than what would normally occur. Over time, these forces result in increased airway resistance and hyperinflation of the lung. LVR entails resection of hyperinflated and nonfunctional lung. The lung remaining within the chest is less diseased, and thus the “average” elastic recoil force will rise.53 As the smaller volume of lung tends to expand to fill the remaining pleural space, radial traction on the terminal bronchioles may increase, thus allowing them to stay patent a bit longer through the respiratory cycle and effecting a later closure. Both the aforementioned effects would tend to decrease airway resistance (or increase airway conductance), improve airflow, and ameliorate hyperinflation.27,61
bronchioles earlier in the expiratory cycle than what would normally occur. Over time, these forces result in increased airway resistance and hyperinflation of the lung. LVR entails resection of hyperinflated and nonfunctional lung. The lung remaining within the chest is less diseased, and thus the “average” elastic recoil force will rise.53 As the smaller volume of lung tends to expand to fill the remaining pleural space, radial traction on the terminal bronchioles may increase, thus allowing them to stay patent a bit longer through the respiratory cycle and effecting a later closure. Both the aforementioned effects would tend to decrease airway resistance (or increase airway conductance), improve airflow, and ameliorate hyperinflation.27,61
Chest Wall and Diaphragm
The cycle of inspiration and expiration is controlled by the excursion of two components of the thoracic cavity, the chest wall and the diaphragm. The diaphragm raises and lowers through its cycle of relaxation and contraction and can be thought of as functioning in a piston-like fashion, helping to suck air in and then drive it out of the lung. The chest wall, through its cycle of increasing and decreasing anteroposterior diameter, acts much like a bellows, sucking air in and blowing it out. In patients with end-stage emphysema, the lung becomes hyperinflated, thus distending the chest wall and flattening the diaphragm. The stretching of this respiratory musculature limits its contractility and radically decreases the inhaled and exhaled volumes achieved during the inspiratory and expiratory cycle.
Excision of nonfunctional hyperinflated lung will lead to a smaller overall lung volume. This provides the opportunity for the chest wall to shrink down to a less hyperexpanded position and the diaphragm to resume its more dome-like appearance. Theoretically this return of the chest wall and diaphragm to their more normal shape and position will restore some of the functional capacity and muscular strength, leading to greater volumes of air movement throughout the respiratory cycle. Teschler,59 Benditt,3 and Tscherncko61 have all documented changes in respiratory mechanics after LVR that support this mechanism for improvement.
Hemodynamics
While end-stage emphysema can result in pulmonary hypertension, LVR is not undertaken in patients who have reached this critical stage. Data on cardiac function and pulmonary hemodynamics in patients with end-stage emphysema are scarce. It is known that the increased intrathoracic pressure generated during forceful expiration in patients with end-stage emphysema can lead to decreased pulmonary venous return, and it has also been suggested that such increases may cause transient extrinsic pressure on pulmonary vasculature and thus increase vascular resistance. LVR would theoretically decrease the size of the lung within the chest cavity and, by decreasing airway resistance, would lead to a lowering of the intrathoracic pressure throughout the respiratory cycle. It might therefore improve venous return, decrease pulmonary vascular resistance, and thus improve right heart output and overall cardiac index.
Preoperative Assessment
The criteria necessary for candidacy for LVR include severe heterogeneous emphysema with hyperinflation and adequate cardiopulmonary reserve to allow for recovery following a major operation. Extensive testing is required to identify appropriate candidates for LVR and includes not only imaging studies but also measurements of pulmonary function, cardiac reserve, and exercise capacity.
Imaging Studies
Certainly the first and perhaps the simplest imaging test to be obtained is the chest x-ray. It should demonstrate hyperinflated lungs with an increased anteroposterior diameter and flattened diaphragms as noted on the lateral projection. The absence of these findings or the presence of notably increased markings
suggesting interstitial disease can allow the clinician to exclude potential candidates without the need for further costly and time consuming workup.
suggesting interstitial disease can allow the clinician to exclude potential candidates without the need for further costly and time consuming workup.
A computed tomography (CT) scan is an essential portion of the workup, not only to document the presence of emphysema suitable for surgical intervention but also to rule out the presence of infiltrative processes and/or occult lung cancer. During preoperative LVR assessment, it is not uncommon to identify a new solitary pulmonary nodule undetected on chest x-ray, and such a nodule requires a full workup to rule in or rule out the presence of malignancy. Even when such a nodule is identified and proved to be a cancer, it is not necessarily a contraindication to surgery if the nodule resides in an area of the lung that is to be resected at the time of LVR surgery.15,47
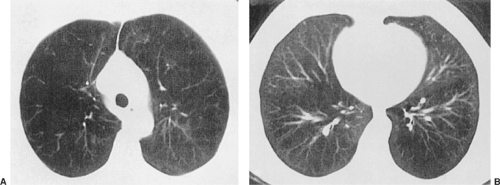
The high-resolution CT is utilized to first determine whether the emphysema is homogeneous in distribution or heterogeneous. For patients with heterogeneous disease, it is then determined whether or not the disease is upper lobe–predominant or lower lobe–predominant. Although Hamacher20 has suggested that patients with homogeneous disease should be considered candidates for surgery, most clinicians would identify the patient with heterogeneous upper-lobe emphysema as the ideal candidate.54 Figure 88-2 demonstrates the CT findings that would be consistent with proceeding to LVR—i.e., relatively severe disease in the upper cuts of the CT scan with relative sparing of the parenchyma in the lower portion.
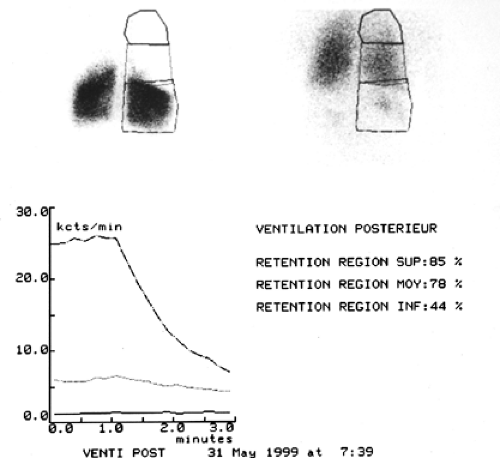
A common clinical evaluation also includes ventilation and perfusion scintigrams obtained on nuclear imaging scans. The ideal findings for LVR would be similar to those in Figure 88-3—e.g., a significant lack of perfusion in the upper lobes bilaterally with persistent gas retention in those same regions. This indicates heterogeneous disease, with the most severe involvement in the upper lobes. This picture of regional, focal lung destruction with relative sparing of the remaining parenchyma identifies both the optimal candidate and the appropriate target for excision.
Pulmonary Function Testing
Full spirometry and lung volume determinations are indicated in all candidates for LVR surgery. The lung volume should be measured via plethysmography rather than the dilution technique, because the former more accurately estimates the degree of lung air trapping and increased residual volume. Optimal candidates are those with severe obstructive disease (forced expiratory volume in one second [FEV1] <40% predicted) without a significant restrictive component or reversible bronchoconstriction. Plethysmography must demonstrate significantly increased total lung capacity (TLC) >120% predicted as well as elevated residual volume (RV) >150% predicted.
Initial testing also includes determination of resting arterial blood gases (PO2 and PCO2). The diffusing capacity (DLCO) is also measured and, as shown later in this text, proves to be an important parameter that can occasionally contraindicate surgical intervention. Ideally, patients who are candidates for LVR have a PO2 >60, a PCO2 <55 mm Hg, and a DLCO >20% predicted.
Maximal Exercise Capacity
The maximal exercise capacity has been shown to play a significant role in predicting prognosis and thus is an important determinant of success in patient selection.41 Two forms of maximal exercise capacity testing have been reported. The simpler of the two is the 6-minute walk test (6MWT), which measures the maximal walk distance achieved by the patient with oxygen supplementation as needed. It is important to undertake this in a reproducible fashion, as varying methodologies will yield different results. Appropriate patient instruction and practice are key to obtaining reliable and reproducible findings.55 In most early clinical series, a 6-minute walk distance of at least 150 m was the lower limit for operative candidacy; patients unable to achieve this minimal requirement were felt to be too disabled to withstand the rigors of surgery.
The second and perhaps more important test is the maximal exercise capacity as determined by incremental symptom-limited exercise utilizing a cycle ergometer. This test is done utilizing oxygen supplementation and will yield the maximum number of watts obtainable by any given subject. Results must be gender-corrected and will allow for categorization into groups with high and low exercise capacity.
Cardiovascular Workup
Patients with end-stage emphysema are clinically fragile and do not tolerate complications following LVR. Because of this, significant cardiac disease is felt to be a contraindication to LVR and potential candidates are carefully screened for evidence of coronary disease using a pharmacologically induced stress test (i.e., dobutamine infusion) with both electrocardiographic monitoring and radionuclide scanning to assess for ischemic redistribution. Doppler echocardiography is routinely used to rule out significant cardiomyopathy and valvular heart disease. Early in the clinical experience, Doppler echocardiography was utilized to try to identify those patients with pulmonary hypertension, because such patients were felt to have an elevated risk of mortality. However, Fisher and coworkers17 documented relatively mediocre sensitivity (60%) and specificity (74%) when echocardiography was utilized in this fashion. Patients in whom pulmonary hypertension is suspected on the basis of signs, symptoms or radiographic findings should therefore undergo right heart catheterization to determine whether pulmonary pressures contraindicate LVR.
Patient Selection
Tables 88-1 and 88-2 outline the inclusion and exclusion criteria that were utilized for the National Emphysema Treatment Trial (NETT). These same criteria continue to be utilized by most surgeons at present and should form the foundation for patient selection. First and foremost is the presence of pulmonary hyperinflation due to obstructive lung disease. Radiographic changes on a plain film (diaphragmatic flattening, increased anteroposterior diameter) provide a good first screening test for patients initially seen in the office. Although there is no absolute upper age limit, most practitioners would prefer to be operating on patients ≤70 years of age. Abstinence from cigarette smoking for a period of at least 6 months is a requirement for consideration of LVR; in those instances where questions arise regarding abstinence, serum and urinary cotinine levels are liberally utilized to confirm the absence of nicotine use.
Airflow limitations as documented on spirometry should be severe with the FEV1 <40% predicted. Volumetric studies utilizing plethysmography should demonstrate residual volumes >150% of predicted and total lung capacity should be >120% of predicted values. Severe hypoxemia with PO2 <55 or PaCO2 >55 should serve as warning signs and are contraindications to LVR.
The imaging selection criteria are based on high-definition CT assessment as well as perfusion and ventilation nuclear imaging of the lung.19 The CT scan is assessed in the upper portion, the midpoint, and lower portion of the lung. Significant heterogeneity should be identified, with the most severe disease being at the apices of the lung and relative sparing of the parenchyma in the middle and lower portions (Fig. 88-2). The ideal candidate will also demonstrate characteristic changes on both the ventilation and the perfusion portions of the lung scan (Fig. 88-3). The perfusion scan should demonstrate poor or absent perfusion in the apices of the lungs, with persistence of ventilation at the bases. The ventilation scan generally indicates areas of gas retention in the upper portion of the lung, with relatively brisk or normal washout of the xenon gas in the bases. This combination of CT and nuclear image findings demonstrate that the apices
of the lung have been destroyed, are relatively nonfunctional, and thus may be safely sacrificed during LVR surgery.
of the lung have been destroyed, are relatively nonfunctional, and thus may be safely sacrificed during LVR surgery.
Table 88-1 Inclusion Criteria (Patients Must Meet All Criteria to Participate | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Finally, the patient’s functional and nutritional status must be such that he or she will tolerate a major surgical insult. An unplanned weight loss of 10% of the patient’s weight over a 3-month period prior to surgery indicates ongoing catabolism and is a relative contraindication to surgery. Body-mass index (BMI) should be ideally between 25 and 35 and a 6-minute walk distance of ≥140 m after rehabilitation is required. Patients falling outside these parameters are felt to have an elevated risk of morbidity or mortality and thus are poor candidates for LVR surgery.
Table 88-2 Exclusionary Criteria (Presence of Any Criterion Makes the Patient Ineligible) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Pulmonary Rehabilitation
Most programs insist on a 6- to 8-week course of pulmonary rehabilitation prior to LVR in order to optimize the patient’s physical condition and thus avoid any unnecessary morbidity or mortality from the operation itself. Rehabilitation comprises a number of elements, including nutritional counseling, psychosocial counseling, and skill training with regard to breathing strategies and management of anxiety. The exercise component includes maneuvers to improve both strength and flexibility as well as to maximize lower- and upper-body aerobic capacity. This entails upper-body weight training as well as treadmill work with supplemental oxygen as necessary.
The NETT provided a unique opportunity to assess the clinical effects of rehabilitation in a large selected patient population. Ries51 reported that in the NETT, there were significant improvements that occurred with regard to exercise capacity whether measured by cycle ergometry with maximal work (8.6% improvement p <0.001) or by 6-minute walk (6.6% improvement p <0.001). In addition, rehabilitation resulted in significant improvements in quality of life, whether measured by the St. George’s Respiratory Questionnaire or the SF36 scale.
The NETT did not contain a randomization arm with regard to rehabilitation; thus all patients underwent preoperative conditioning. It is therefore impossible to state definitively that preoperative rehabilitation is an absolute necessity. However, expert opinions suggest that a period of preoperative conditioning is likely to decrease perioperative morbidity as well as speed postoperative recovery, and preoperative rehabilitation is currently the standard of care.
Surgical Techniques
Parenchymal Ablation
Early in the history of LVR, multiple techniques were being utilized. Laser ablation was first recommended by Wakabayashi,63 but two clinical series published by McKenna32 and Hazelrigg96 compared laser ablation and stapling and found the latter to be superior. Thus laser ablation has been virtually abandoned since that time.
One additional reported technique was a nonresecting technique that involved defunctionalizing a portion of lung by tightly rolling or plicating it. The lung was then stapled in this configuration to prevent reinflation. This technique was championed by Swanson58 but never gained widespread popularity, and this technique too has disappeared from practice.
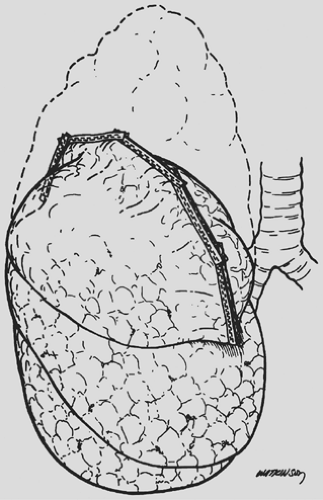
Currently the technique utilized by virtually all surgeons is a stapled resection of the lung, most commonly removing the apex of the upper lobe. This is performed utilizing sequential firings of a surgical stapler with intent of resecting approximately half to two-thirds of the upper lobe of the lung (Fig. 88-4). Staple-line reinforcement is commonly performed utilizing buttresses fashioned either from nonabsorbable (bovine pericardium, polytetrafluoroethylene) or absorbable material (collagen, trimethylene carbonate polymer). Although there are conflicting results as to the efficacy of staple-line buttressing with regard to control of air leak, most surgeons routinely utilize this adjunct.23,39,57
Unilateral Versus Bilateral Approach
Early during the modern evolution of LVR surgery, there was a controversy regarding whether to perform the procedure unilaterally or bilaterally. Cooper8 and other investigators performing median sternotomy routinely performed simultaneous bilateral procedures. Those performing a thoracoscopic LVR surgery initially performed unilateral procedures.26,33,34,43 This rapidly evolved to a simultaneous bilateral thoracoscopic procedure, but—not surprisingly—it was clear from early results that the morbidity of a bilateral thoracoscopic procedure exceeded that of a unilateral procedure. Thus the question arose as to whether LVR should be done unilaterally, bilaterally in a sequential fashion, or in a simultaneous bilateral procedure. Studies by McKenna33,34 and Argenziano1 suggested that a bilateral LVR surgery yielded significantly greater improvements in spirometric indices than did unilateral procedures. Interestingly, the former author noted no increased morbidity for the
bilateral approach, while the latter author noted significantly increased morbidity for bilateral procedures. Naunheim44 noted that bilateral procedures led to a higher incidence of prolonged air leak, pneumonia, and arrhythmia. Lowdermilk31 compared functional and spirometric results of unilateral and bilateral approaches for thoracoscopic LVR surgery in a multi-institutional review. This study confirmed that the bilateral approach resulted in superior improvement in FEV1 and RV (residual volume) as well as improved quality of life and dyspnea indices. It is interesting to note, however, that none of the reports noted above identified a significant improvement in exercise capacity or decreased oxygen utilization when unilateral and bilateral procedures were compared.
bilateral approach, while the latter author noted significantly increased morbidity for bilateral procedures. Naunheim44 noted that bilateral procedures led to a higher incidence of prolonged air leak, pneumonia, and arrhythmia. Lowdermilk31 compared functional and spirometric results of unilateral and bilateral approaches for thoracoscopic LVR surgery in a multi-institutional review. This study confirmed that the bilateral approach resulted in superior improvement in FEV1 and RV (residual volume) as well as improved quality of life and dyspnea indices. It is interesting to note, however, that none of the reports noted above identified a significant improvement in exercise capacity or decreased oxygen utilization when unilateral and bilateral procedures were compared.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree