Lung Transplantation
Felix G. Fernandez
G. Alexander Patterson
Historical Review
Early Phase
During the early 1950s, Metras78 in France and Hardin and Kittle51 in the United States reported successful canine lung transplantation. During that decade, many surgical techniques were developed that have proved useful in human lung transplantation. Hardy and associates reported the first human lung transplantation in 1963.52 Although their patient succumbed after 18 days, their brief success demonstrated the technical feasibility of the operation and stimulated worldwide interest in pulmonary transplantation.
During the next 15 years, about 40 clinical lung transplant operations were performed in centers around the world. None of these procedures was successful. The only recipient actually discharged from the hospital was a 23-year-old patient of Derom and colleagues.36 This patient left the hospital 8 months after transplantation and died a short time thereafter as a result of chronic rejection, sepsis, and bronchial stenosis. Most patients in this era died within 2 weeks of lung transplantation as a result of primary graft failure, sepsis, or rejection. The most frequent cause of death beyond the second week was bronchial anastomotic disruption. This problem of bronchial anastomotic dehiscence stimulated the interest of investigators in a number of surgical laboratories. Lima and colleagues71 in Toronto demonstrated that high doses of corticosteroids (2 mg/kg per day), necessary for immunosuppression in that era, had an adverse effect on bronchial anastomotic healing. The same group, as reported by Morgan and associates,82 also demonstrated that the ischemic donor bronchus could be revascularized within a few days by a pedicled flap of omentum. The omental pedicle provided new collateral circulation to the ischemic bronchus and the omentum itself also had a potential benefit in containing an anastomotic dehiscence in the event of partial disruption.
During the same interval, the new drug cyclosporine demonstrated impressive immunosuppressive properties and eliminated the routine need for early high-dose corticosteroids. Furthermore, it was also demonstrated by the Toronto group, and reported by Goldberg and colleagues,47 that cyclosporine had no adverse effect on bronchial anastomotic healing.
Reitz and associates97 of Stanford reported initial clinical experience with combined heart–lung transplantation in patients with pulmonary vascular disease. This experience demonstrated conclusively that the transplanted human lung could provide acceptable long-term function. Yet by 1983, successful isolated lung transplantation had not yet been achieved. Satisfactory patient selection remained the final obstacle to successful clinical lung transplantation. The Toronto group reasoned that end-stage respiratory failure from pulmonary fibrosis would provide the ideal physiologic conditions for single lung transplantation. The increased resistance to both perfusion and ventilation of the native lung would preferentially direct perfusion and ventilation to the transplanted lung. By careful recipient selection and strict adherence to rigid donor criteria, Cooper and colleagues achieved the first successful single lung transplantation in 1983 in a 58-year-old man with idiopathic pulmonary fibrosis, as reported by the Toronto Lung Transplant Group.115
The subsequent development of an experimental and clinical en bloc bilateral lung replacement technique by Dark26 and one of us (G.A.P.) and associates90 enabled application of bilateral lung replacement in patients for whom single lung transplantation was not appropriate. Although this procedure did have the definite attraction of preservation of the recipient heart, it was technically complex. Double lung transplantation with a tracheal anastomosis was associated with a high incidence of complications, notably donor airway ischemia, as the authors described,91 and cardiac denervation, as reported by Schaefers and colleagues.102
Current Status
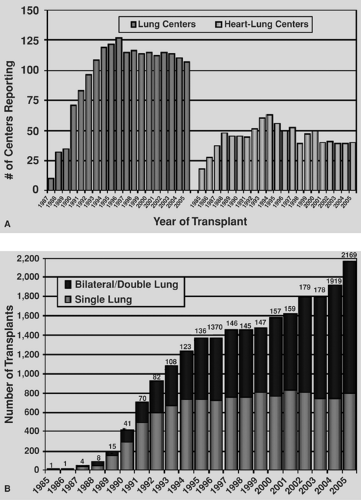
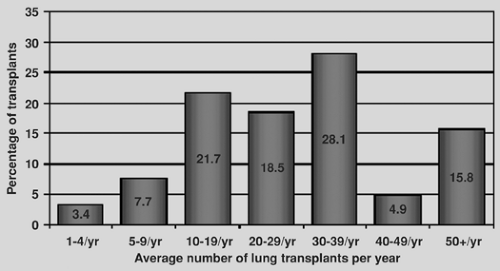
As a treatment strategy for end-stage lung disease, transplantation has matured significantly in the past decade. In 1988, there were only a handful of lung transplantation programs worldwide. Currently, there are experienced lung transplant programs in most western nations. Additionally, eastern nations are beginning to develop an experience as well, most notably in Japan and China. The International Society of Heart and Lung Transplantation (ISHLT) Registry has evolved into a major repository of data for the international lung transplant community.116 Recent figures from the ISHLT registry reproduced in Figure 98-1A demonstrate that the number of lung transplant centers worldwide has remained stable over the past decade and that most centers perform between 10 and 39 transplantations per year, with the distribution of transplants by center volume shown in Figure 98-1B. The number of lung transplants by year and procedure type is shown in Figure 98-2.
Recipient Selection and Organ Allocation
General Considerations
General selection criteria are listed in Table 98-1 and are summarized by Maurer and colleagues.74 Patients who have coexisting dysfunction involving another organ system are not eligible for transplantation, a limitation that especially affects patients in older age groups. In general, transplant centers do not accept patients >65 years of age. The ISHLT Registry lists advanced recipient age as a specific predictor of increased medium and long term posttransplant mortality.116 In addition, patients with a history of malignant disease within the prior 5 years are
generally not eligible for pulmonary transplantation. A potential exception is a patient with bilateral bronchoalveolar carcinoma. Etienne and colleagues39 have reported a single long-term survivor with this therapy, and Zorn and associates122 have reported a favorable experience in a small number of patients. The patient with a recent extrathoracic malignancy judged to be cured might be considered. Patients with serious psychological dysfunction should not be considered candidates for pulmonary transplantation. Few groups evaluate patients who continue to smoke.
generally not eligible for pulmonary transplantation. A potential exception is a patient with bilateral bronchoalveolar carcinoma. Etienne and colleagues39 have reported a single long-term survivor with this therapy, and Zorn and associates122 have reported a favorable experience in a small number of patients. The patient with a recent extrathoracic malignancy judged to be cured might be considered. Patients with serious psychological dysfunction should not be considered candidates for pulmonary transplantation. Few groups evaluate patients who continue to smoke.
Previous thoracic surgery is not a specific contraindication to pulmonary transplantation, although resulting adhesions and anatomic distortion do complicate a subsequent transplant procedure. Patients receiving high-dose corticosteroid therapy (≥20 mg prednisone) are not eligible for lung transplantation, as a well-documented negative influence on bronchial healing and susceptibility to postoperative infection have been demonstrated. However, low- or moderate-dose steroid therapy does not result in an increased incidence of bronchial anastomotic complications. Ventilator dependency is not a specific contraindication to transplantation; however, the ISHLT Registry experience identifies recipient ventilator dependence as a risk factor for increased mortality.116
All patients considered for transplantation, except those with primary pulmonary hypertension or Eisenmenger’s syndrome, participate in a monitored exercise rehabilitation program while awaiting transplantation. Virtually all patients experience an improvement in strength and exercise tolerance without any measurable change in pulmonary function. This improved endurance better enables patients to withstand the rigors of a transplant procedure and subsequent convalescence.
Table 98-1 Recipient Selection Criteria | |
|---|---|
|
Traditionally, lung allocation in the United States was based on time on the wait list, regardless of medical urgency or deterioration in medical condition. Problems with this system included ease of gaming by early listing of potential recipients to accrue wait time and a favoring of recipients well enough to survive on the transplant list, while those who may benefit most risk death while waiting. An ideal system balances organ allocation based on clinical necessity while selecting recipients able to recover from a transplant operation. Recently, the United Network for Organ Sharing (UNOS) Thoracic Organ Committee revised the listing algorithm by assigning each patient a lung allocation score (LAS), based on the immediate need for transplant and the probability of posttransplant survival. This led UNOS to rearrange its wait list for adult lung transplantation in 2005 to one based on the LAS score. Details of the new allocation system are found on the official Organ Procurement and Transplantation Network Web site (http://www.optn. org/PoliciesandBylaws/policies/pdfs/policy_9.pdf [accessed November 24, 2007]). Briefly, the LAS score is calculated by estimating waitlist urgency, defined as the expected number of days that could be lived without a transplant (Table 98-2), and posttransplantation survival, defined as the expected number of days lived during the first year after transplantation (Table 98-3). The transplant benefit measure is then derived by subtracting the waitlist urgency from the posttransplantation survival to obtain the raw allocation score (calculated in days). This score is normalized to the LAS on a scale of 1 to 100. Those with the highest scores are listed first for transplantation. Factors used to predict risk of death and posttransplantation survival are regularly reviewed by the Thoracic Organ Transplantation Committee and updated as appropriate.
Table 98-2 Factors Used to Predict Risk of Death While on the Transplant Waiting List | ||
|---|---|---|
|
Specific Considerations Based on Diagnosis
Obstructive Lung Disease
Obstructive lung disease, notably emphysema and alpha1-antitrypsin deficiency, is the most common indication for lung transplantation, accounting for 46% of the adult lung transplantations included in the 2007 Registry of the UNOS/ISHLT, as reported by Trulock and colleagues.116 Most patients have deteriorated to a point at which oxygen supplementation is required by the time of listing, typically slightly greater than 4 L/min of oxygen. A forced expiratory volume in 1 second (FEV1) of well under 1 L at about 15% of predicted normal values is observed in these patients. Generally, this patient group usually has a stable course while awaiting lung transplantation. This stability affects the LAS of patients within this group, resulting in a lesser percentage of transplants for obstructive lung disease since the implementation of this allocation system.
Although obstructive lung disease is the most common indication for which lung transplantation is performed, the ideal operative procedure for these patients remains to be defined. There are currently two surgical therapies aimed at crippling end-stage emphysema: lung transplantation and lung volume reduction surgery. Early reports of the results of lung volume reduction surgery suggest that this procedure can offer significant improvement in the symptoms of severe emphysema and a measurable improvement in overall functional status. In a retrospective comparison of volume reduction and transplantation for emphysema, Gaissert and associates reported a lower early and late mortality for patients receiving volume reduction: 3.0% for volume reduction, 10.2% for single lung transplantation, and 16% for bilateral lung transplantation.43 In the same study, the percent improvement of the FEV1 at 12 months after operation was 83% for the volume reduction group, 212% for the single lung transplant group, and 518% for the bilateral lung transplant group. Meyers and colleagues, from the authors’ institution, further investigated the outcome of lung reduction specifically in patients considered eligible for transplantation; they found satisfactory results without evidence of excess mortality in the subsequent lung transplantation.81
Table 98-3 Factors That Predict Survival After Lung Transplantation | ||
|---|---|---|
|
However, the authors favor a meticulous selection process in which both transplant and volume reduction are considered, with the best option being selected for each patient. Patients with ideal circumstances for lung volume reduction surgery—such as hyperinflation, heterogeneous distribution of disease, FEV1 >20%, and a normal PCO2—are offered lung volume reduction surgery, whereas patients with diffuse disease, low FEV1, hypercapnia, and associated pulmonary hypertension are directed toward transplantation. Lung volume reduction surgery has not been a satisfactory option for patients with alpha1-antitrypsin deficiency.
Functional outcomes of single and bilateral lung transplantation are discussed below. Generally, for young patients, particularly those with alpha1-antitrypsin deficiency, the authors prefer bilateral sequential lung transplantation. The bilateral option is also more attractive in larger recipients for whom an oversized single lung donor would be difficult to obtain. For smaller recipients, single lung transplantation offers a more attractive option, particularly when an oversized donor lung can be grafted. This decision of single versus bilateral transplantation for emphysema is controversial and requires consideration of benefit to an individual recipient versus global benefit from a limited supply of available donor lungs.
Septic Lung Disease
Cystic fibrosis (CF) is the most frequently encountered disease in this category. It is a common inherited disorder resulting in diffuse bronchiectatic destruction of both lungs. Without transplantation, the overwhelming majority of patients die as a result of progressive respiratory failure in the second or third decade of life. As reported in the 2007 ISHLT Registry, CF is now the most common indication for bilateral lung transplantation and the third most common indication for lung transplant in general.116 The most reliable predictors of life expectancy in CF patients were published by Kerem and associates.63 An FEV1 of less than 30% predicted, elevated PaCO2, requirement for supplemental oxygen, frequent admissions to the hospital for control of acute pulmonary infection, and failure to maintain weight are reliable predictors of early mortality in these patients. At this stage of disease, patients with CF usually have a rapidly progressive downhill course. Up to one-third of CF patients listed for transplantation die on the waiting list.103 To offset this high waitlist mortality,
some of the factors listed above have been incorporated into the LAS.
some of the factors listed above have been incorporated into the LAS.
Fibrotic Lung Disease
Pulmonary fibrosis or restrictive lung disease includes patients with idiopathic pulmonary fibrosis (IPF), pulmonary fibrosis of other etiologies, sarcoidosis with elevated pulmonary artery pressures, and obliterative bronchiolitis (not retransplant cases). In the past, this category represented one of the least common indications for single lung transplantation; however, the 2007 ISHLT Registry report indicated that IPF was the second most common indication for single lung transplantation and the third most common for bilateral lung transplantation.116 In the authors’ experience, candidates for transplantation have classic restrictive findings on spirometry, with a mean forced vital capacity (FVC) of 1.35 L and an FEV1 of 1.14 L.79 All were using supplemental oxygen and demonstrated marked impairments in exercise tolerance. Moderate pulmonary hypertension is common in these patients. Patients with pulmonary fibrosis who require a transplant have been observed to have a rapid downhill course, which affects the ability of these patients to advance on the waiting list.
Pulmonary Vascular Disease
Patients with pulmonary vascular disease were once thought to require combined heart–lung transplantation. Fremes and colleagues42 performed a successful single lung transplantation in a patient with a patent ductus arteriosus and Eisenmenger’s syndrome. Subsequently, several centers have reported that right ventricular function improves immediately after transplantation and is maintained at long-term follow-up.21,77 Reliable predictors of early death in patients with primary pulmonary hypertension have been established, including mean pulmonary artery pressures >60 mm Hg, syncopal episodes, clinical evidence of right ventricular failure, significant elevation of central venous pressure, and depression of the cardiac index.24 Among the authors’ first 34 patients undergoing single lung transplantation for pulmonary vascular disease, the New York Heart Association functional status was class III or IV in every patient.88
In the late 1980s and early 1990s, lung transplantation was the only effective therapy for patients with end-stage primary pulmonary hypertension (PPH). During that period there was a very high death rate among patients with PPH waiting for lungs.45 Subsequent studies demonstrate that continuous intravenous (IV) infusion of epoprostenol, a vasodilating prostaglandin, produces a symptomatic and hemodynamic benefit as well as improved survival for patients with PPH.6 This has led to fewer patients being referred for transplantation and the absolute number of patients transplanted annually for PPH has decreased dramatically. More recently, a stepwise regimen of medical therapy, including oral and inhaled prostanoids followed by IV therapy followed by lung transplantation, has been developed.25 It is felt that this can improve survival from the time of PPH diagnosis and decrease mortality while on the transplant wait list. Patients with Eisenmenger’s physiology, on the other hand, have a less predictable rate of deterioration despite equivalent degrees of pulmonary hypertension. The development of progressive right ventricular failure is utilized as a selection criterion in these patients.
Infant and Pediatric Recipients
Lung transplantation was expanded to the pediatric population in a judicious manner in the late 1980s. The number of lung transplantations for recipients <18 years of age was 67 for the year 2004, as reported by the ISHLT.118 This number has remainded stable over the past decade and represents a small proportion of the total lung transplant procedures performed. The most common indication for lung transplantation in infants (<1 year of age) is congenital heart disease, representing 32% of the procedures performed in this age group between 1991 and 2004. Other indications in this age group include PPH, pulmonary vascular disease, and pulmonary alveolar proteinosis. In the group between 1 and 10 years of age, 54% of lung transplants were performed for cystic fibrosis during 1991–2006; other indications include PPH, congenital heart disease, and retransplantations for obliterative bronchiolitis. CF is the predominant indication for lung transplantation in teenagers between 11 and 17 years of age, accounting for about 70% of procedures performed in this age group. The lung allocation system has recently been revised, and currently lungs are allocated to potential recipients <12 years of age based on waiting time on the transplant list. Alternatively, potential recipients between 12 and 17 years of age are prioritized based on their LAS, which is derived from their predicted survival with or without a transplant.37
Donor Lung
Rapid progress in transplantation has resulted in a shortage of suitable allografts for all organs. This problem is particularly significant for lung transplantation, since only 20% of otherwise suitable organ donors have lungs satisfactory for transplantation according to the criteria listed in Table 98-4. Most of the conditions that result in brain death (trauma, spontaneous intracerebral hemorrhage) lead to significant pulmonary parenchymal pathologic change because of lung contusion, infection, aspiration, or neurogenic pulmonary edema.
Satisfactory gas exchange is imperative for donor lungs. This parameter can be confirmed by noting a PAO2 of >300 mm Hg with an inspired oxygen concentration of 100% and 5 cm H2O peak end-expiratory pressure (PEEP). A PAO2-to-FIO2 ratio of 300 or greater provides adequate evidence of satisfactory gas exchange. A donor chest radiograph taken shortly before harvest must reveal clear lung fields. Bronchoscopic assessment at the donor institution often reveals mucopurulent secretions
that might contain a variety of microorganisms. This finding is commonly observed and is not a specific contraindication to pulmonary transplantation if the donor is otherwise suitable. Bronchoscopic evidence of aspiration or frank pus in the airway, however, is a definite contraindication to transplantation of that lung.
that might contain a variety of microorganisms. This finding is commonly observed and is not a specific contraindication to pulmonary transplantation if the donor is otherwise suitable. Bronchoscopic evidence of aspiration or frank pus in the airway, however, is a definite contraindication to transplantation of that lung.
Table 98-4 Ideal Lung Donor Selection Criteria | |
|---|---|
|
Donor and recipient ABO blood compatibility is essential. Donor and recipient histocompatibility antigen (HLA) matching is not currently performed. There is controversy regarding the importance of HLA matching, and no data in the literature support its impact on subsequent graft function. Furthermore, any delay in donor harvest to conduct HLA matching places the donor lungs at risk for deterioration. Unfortunately, satisfactory preservation strategies are not available to permit postprocurement tissue matching. The authors prefer to use cytomegalovirus (CMV)-negative donors for CMV-negative recipients whenever possible.
Size matching between donor and recipient is a significant consideration. Acceptable size matching depends on the nature of the recipient’s lung disease and the type of transplant anticipated. The most reliable method of size matching predicts donor and recipient lung volumes using standard nomograms based on age, sex, and height, used by all pulmonary function laboratories to obtain predicted lung volumes. In patients undergoing single lung transplantation for obstructive lung disease, the authors attempt to place allografts with 15% to 20% greater volume than the recipient predicted lung volume. Implantation of a large allograft is easily achieved in a patient with obstructive lung disease because of the enormous size of the recipient pleural space. In patients with pulmonary fibrosis or pulmonary vascular disease, however, the pleural spaces are reduced or normal in size, respectively. It is therefore inadvisable to oversize these patients to an excessive degree. In patients undergoing bilateral lung replacement, the authors prefer to match the donor lung volumes to the lung volume or anticipated lung dimensions that the recipient would possess in the absence of lung disease. Oversizing a bilateral recipient may produce hemodynamic difficulties on closure of the chest at the termination of the procedure.
Certain circumstances allow relaxation of the typically strict donor selection criteria. A minor degree of pulmonary infiltrate may be accepted in donor lungs being used for a bilateral transplantation. The authors analyzed 133 consecutive donor lungs and identified 37 with marginal quality, as judged by arterial blood gas analysis and radiographic assessment. The marginal donors provided postoperative function equivalent to those judged excellent.110 Occasionally, a donor is identified with marginal gas exchange and radiographic evidence of a unilateral pulmonary infiltrate. In several such situations, the authors have made intraoperative donor assessments with ventilation and perfusion only to the seemingly normal donor lung, judged that lung to be acceptable, and conducted a successful unilateral transplantation.95
The ability of pulmonary parenchymal cells to continue aerobic cellular metabolism by relying on the oxygen within the alveoli makes the lung potentially the ideal organ for transplantation after cessation of circulation, so-called donation after cardiac death (DCD). Animal data show that lungs harvested as long as 4 hours after death from dogs whose hearts were not beating continue to have acceptable function.98 Several clinical series now support these animal data. A series from Spain reported excellent early allograft function with no ischemia/reperfusion injury even after 11 hours of total ischemia, and outpatient follow-up to 13 months after transplantation documented adequate lung function and quality of life.44 More recent follow-up of this experience demonstrates a 3-year survival of 58% in 17 recipients of lungs from non–heart-beating donors.48 Steen and colleagues107 from Sweden have advocated extracorporeal perfusion and ex vivo assessment of donor lung function in non–heart-beating donors, with encouraging results in animal models. Given these promising results, the authors have begun to accept organs from non–heart-beating donors, with preliminary results similar to those seen with cadaveric donors.
Living Donor Transplantation
Living-donor lung transplantation was pioneered by the group at the University of Southern California in an attempt to expand the donor pool. Two donors, usually family members, undergo right lower and left lower lobectomy, respectively. The pulmonary artery and vein of the allograft are cannulated and flushed with pulmonoplegic solution both antegrade and retrograde. Cold ischemic times are relatively short compared with those of cadaveric lung transplants. The recipient operation is usually performed through a transverse thoracosternotomy incision with the use of cardiopulmonary bypass (CPB). Prolonged postoperative ventilatory support may help decrease atelectasis. Recipients of living donor lobes may be at an increased risk for development of pulmonary edema postoperatively because the entire cardiac output flows to only two lobes. Furthermore, because these patients typically have higher outputs from their chest tubes owing to space considerations, it has been the practice of the group at the University of Southern California to keep the chest tubes in place for a prolonged period. Between 1993 and 2003, a total of 123 patients underwent living lobar lung transplantation at the University of Southern California, which represents the largest single-center experience.106 This procedure was predominantly employed for patients with CF, and one-third of the recipients were children. The 1-, 3-, and 5-year survival rates were 70%, 54%, and 45%, respectively, comparable to outcomes for bilateral cadaveric lung transplantation. Infection was the major cause of recipient mortality, and the freedom from bronchiolitis obliterans syndrome in their adult population was 76% at 5 years, better than for recipients of bilateral cadaveric grafts. Date and colleagues28 from Okayama University in Japan have reported an experience of 43 living lobar transplants. Indication for transplant was pulmonary hypertension in 35%, pulmonary fibrosis in 28%, and obliterative bronchiolitis in 16%. Results in this series were excellent, with no deaths in the first 3 years posttransplant and a 5-year survival of 87%.
Lung Preservation
Lung preservation has been the focus of intense laboratory interest since the start of lung transplantation. This work has been reviewed in detail.32,33 Clinical pulmonary preservation has progressed considerably since the Toronto Group initially carried out unilateral lung transplantation using donor lungs harvested in an atelectatic state and stored by topical hypothermic immersion.113 Minor differences occur in the preservation
strategies of most clinical lung transplant programs, but the basic principles remain the same.
strategies of most clinical lung transplant programs, but the basic principles remain the same.
After systemic donor heparinization and just before circulatory arrest, a pulmonary vasodilator is used, usually administered by direct bolus injection into the pulmonary artery. The authors use 500 μg of prostaglandin E1 (PGE1). Pulmonary arterial flush is then achieved after aortic clamping with the lungs in a state of moderate inflation at an FIO2 greater than room air. The authors use a low-potassium dextran solution, Perfadex (Vitro Life, Goteburg, Sweden) as a pulmonary flush solution instead of an intracellular solution such as Euro-Collins or the University of Wisconsin solution. This is based on the authors’ clinical observation that the use of high-potassium solutions results in a higher degree of ischemia/reperfusion injury and primary graft dysfunction. This observation is corroborated by both laboratory and clinical data.15,65,83 After extraction, the lung allograft is immersed in iced crystalloid solution and maintained in a semi-inflated state during transport. This technique results in reliable allograft function, with ischemic times of up to 6 hours. Occasionally, the ischemic time has been extended, particularly for the second lung of a bilateral sequential transplantation, in which the ischemic time has reached 8 to 10 hours with satisfactory subsequent function.
The delivery of the pulmonary flush solution is performed in an antegrade manner through the main pulmonary artery. The addition of retrograde perfusion has been described by Varela and colleagues,117 and this technique has been successfully utilized. There are potential benefits of improved distribution of flush solution with retrograde delivery. Additionally, for donors at high risk for acute pulmonary thromboembolism, retrograde flush can identify and remove embolic clot from the pulmonary artery.
Inflation
The state of lung inflation during pulmonary artery flush and storage probably has a significant impact on posttransplantation pulmonary function. Previous work demonstrated that lungs flushed and stored in a hyperinflated state produced more reliable posttransplantation function.94 However, when this was adopted as a policy of donor hyperinflation in the clinical program, the authors noted a disturbing incidence of allograft dysfunction. Further, evidence from the laboratory using a rabbit model suggests that hyperinflation produces increased pulmonary capillary permeability. For this reason, the authors recommend that lungs be flushed and stored in a state of moderate inflation consistent with normal end-tidal inspiration.50
Temperature of Flush and Storage
Clinical lung transplantation programs generally use pulmonary artery flush temperatures of 1°C to 4°C. After extraction, lungs are immersed in crystalloid solution and packed in ice, resulting in their storage and transport at about 1°C. Some investigators have shown that a more moderate degree of hypothermia (10°C) results in superior lung function. The authors demonstrated this principle in the laboratory in an in vitro rabbit lung perfusion model119 as well as a standard model of canine left lung allotransplantation and bilateral baboon lung transplantation.108 In a previous study, however, Mayer and colleagues75 were unable to show a difference between storage at 4°C versus 10°C in canine left lung allografts. Although there are some studies suggesting improved lung function in small animal models after storage at even higher temperatures, hypothermic flush and storage remains the standard of care at virtually all lung transplant centers.120
Pharmacologic Manipulation
In addition to the apparent benefit gained when prostaglandins are administered before pulmonary artery flush,75 evidence suggests that infused prostanoids are useful in the early posttransplantation period. Matsuzaki and associates73 have shown that PGE1 infusions decrease reperfusion injury after 2 hours of warm ischemia in a rabbit lung model. The authors continued this work, demonstrating that PGE1 improved canine lung allograft function after an 18-hour ischemic period.2
There is mounting evidence suggesting that oxygen free radicals are important mediators of ischemia/reperfusion injury in the lung. A number of antioxidant interventions, including enzymatic (superoxide dismutase, catalase, or glutathione peroxidase) and nonenzymatic (allopurinol, glutathione, dimethyl- thiourea, and lazaroid) agents have been shown to reduce lung reperfusion injury; some of these have been used with success in clinical lung transplant programs.
It is well established that levels of endogenous nitric oxide, a potent vasodilator, drop after ischemia and reperfusion of lung grafts. Several studies have been conducted testing the effect of inhaled nitric oxide on graft function. Although still controversial, the majority of studies seem to indicate that inhaled nitric oxide offers no benefit in reducing graft dysfunction related to ischemia and reperfusion.3,76,92
Technique
Donor Lung Extraction
Donor extraction technique remains essentially unchanged from that reported by Sundaresan and associates.109 After the lung extraction team’s arrival at the donor hospital, it is important for the team to assess the chest radiographs and to perform fiberoptic bronchoscopy. Final assessment is made by gross inspection of the lungs, which are exposed by median sternotomy performed in conjunction with the midline laparotomy for extraction of abdominal organs.
The abdominal organs are prepared for extraction by the responsible surgical teams. Both venae cavae are encircled within the pericardium. The aorta is mobilized and encircled. Care must be taken to avoid injury to the right main pulmonary artery lying immediately posterior to the superior vena cava and ascending aorta. It is not necessary to dissect the main pulmonary artery. The donor is heparinized. In the case of concomitant cardiac retrieval, a cardioplegia cannula is placed in the ascending aorta. A large-bore pulmonary flush cannula then is placed in the main pulmonary artery immediately proximal to its bifurcation. PGE1, 500 μg, is administered directly into the main pulmonary artery, which produces an immediate fall in systemic pressure. At this point, ligation of the superior vena cava is performed and the inferior vena cava is vented into the chest to allow decompression of the right heart. The aorta is then cross-clamped, and cardioplegic solution is initiated. Cardioplegia is
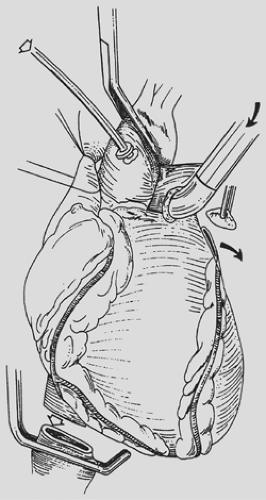
vented through the inferior vena cava, which is divided immediately above the previously placed clamp. With the lungs continuously ventilated, pulmonary artery flushing is achieved with 60 mL/kg of Perfadex solution delivered at a pressure of 30 cm H2O. This solution is vented through the amputated tip of the left atrial appendage (Fig. 98-3). Cold effluent is allowed to collect in both pleural spaces. Topical hypothermia is supplemented by crushed ice.
vented through the inferior vena cava, which is divided immediately above the previously placed clamp. With the lungs continuously ventilated, pulmonary artery flushing is achieved with 60 mL/kg of Perfadex solution delivered at a pressure of 30 cm H2O. This solution is vented through the amputated tip of the left atrial appendage (Fig. 98-3). Cold effluent is allowed to collect in both pleural spaces. Topical hypothermia is supplemented by crushed ice.
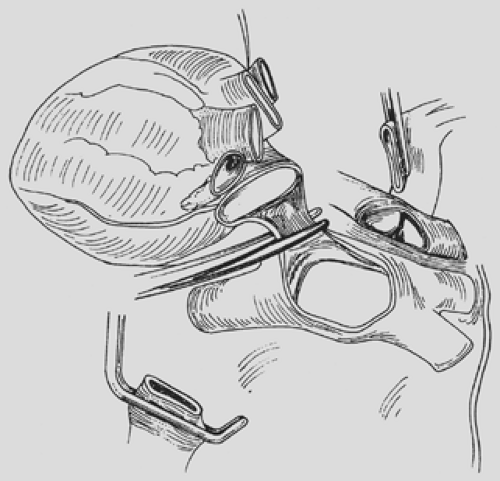
The authors prefer to extract the donor heart in situ. It should be stated emphatically that satisfactory cardiac and bilateral lung grafts can be safely extracted in every situation. The superior vena cava is divided between the previously placed ligatures, once again taking care not to injure the underlying right main pulmonary artery. The aorta is divided superior to the cardioplegia cannula. The main pulmonary artery then is divided through the cannulation site. The heart then is elevated and retracted to the right. The left atrium is opened midway between the coronary sinus and the inferior pulmonary vein. The left atrial incision is continued toward the right. The right side of the left atrial wall is divided, taking care to preserve a rim of atrial muscle on the pulmonary vein side (Fig. 98-4). This step completes the cardiac excision. Before extraction of the lungs, they are flushed retrograde by administering 250 mL of Perfadex solution through each pulmonary vein orifice.
Extraction of the lungs is continued by mobilization and division of the trachea well above the carina. The entire thoracic contents then are extracted from the spine in a caudal direction. The lung allografts are immersed in cold crystalloid solution and transported semi-inflated. Should each lung be used in a different transplantation center, they are separated into distinct allografts at the donor hospital. The donor left main bronchus is divided at its origin with a cutting stapling device to leave the airway to both lungs sealed (Fig. 98-5). Otherwise, the grafts should be transported en bloc for separation immediately
before implantation. On arrival at the recipient hospital, the graft is exposed and kept cold during the remainder of its preparation. The esophagus and aorta are removed, taking care to leave all other soft tissues on the specimen side to maximize bronchial arterial collateral flow to the donor lung.
before implantation. On arrival at the recipient hospital, the graft is exposed and kept cold during the remainder of its preparation. The esophagus and aorta are removed, taking care to leave all other soft tissues on the specimen side to maximize bronchial arterial collateral flow to the donor lung.
The posterior wall of the left atrium is divided, leaving equal atrial cuffs on both sides. The pulmonary artery is divided at its bifurcation (see Fig. 98-5). It is important to separate the pulmonary artery from its pericardial attachments on each side out to the first pulmonary arterial branch. This separation prevents compromise of pulmonary artery caliber distal to the pulmonary artery anastomosis after implantation.
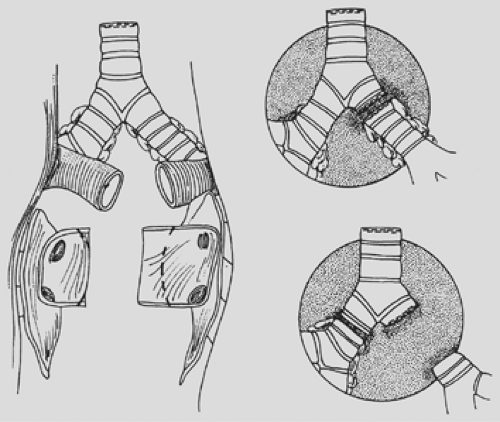
Subcarinal nodes are divided, and the left main bronchus is transected. The left main bronchus is dissected from nodal tissue and divided at a point two rings proximal to the upper lobe orifice. On the right side, excision of the carina usually provides an adequate length (two rings proximal to the upper lobe origin) for subsequent bronchial anastomosis. It is important during dissection of the bronchus to minimize any nodal dissection at the site of bronchus transection in order to maximize retrograde bronchial collateral blood flow to the donor bronchus after transplantation.
Recipient Preparation
Anesthesia
A successful lung transplantation program requires active involvement of expert anesthesiologists familiar with complex cardiothoracic anesthesia techniques, bronchoscopy, and CPB. Full hemodynamic monitoring is required in every patient. The anesthesia team at the authors’ institution routinely use a Foley catheter, central venous line, pulmonary artery Swan–Ganz catheter, and radial and femoral arterial catheters. Also utilized in every patient is a transesophageal echocardiographic probe, but its application is especially critical in patients with severe pulmonary hypertension and coexistent right ventricular dysfunction.
The airway is routinely intubated with a left-sided double-lumen endobronchial tube. In small patients, a single-lumen tube with an endobronchial Fogarty catheter enables independent ventilation; however, this technique does not have the reliability of a double-lumen tube. A single-lumen tube can present difficulties, particularly in a bilateral transplant recipient in whom intraoperative maneuvering of the tube can be troublesome. For small-stature adults or pediatric recipients, a single-lumen tube is occasionally used with the expectation that cardiopulmonary bypass will be used during the explantation and implantation. In patients with CF, thick, tenacious, purulent secretions are continuously expressed into the bronchial lumen during manipulation of the lungs for extraction. It is extremely difficult to evacuate secretions from the airway, particularly through a small-caliber double-lumen endotracheal tube. These secretions can result in impaired ventilation. To minimize this problem, a large-caliber single-lumen tube is placed and, using a flexible fiberoptic bronchoscope, the airway is irrigated and aspirated as much as possible before placement of the double-lumen tube and initiation of the procedure. For patients of small stature, a single-lumen tube must be used. If a bilateral procedure is planned in such patients, CPB is used routinely during extraction and implantation. This is the standard technique for bilateral transplantation in children.72
Single Lung Transplantation
Choice of Side
The authors prefer to transplant the side with the least function as judged by preoperative quantitative nuclear perfusion scans. There is no difference in functional outcome among single lung recipients regardless of transplant side.70 If CPB is anticipated, as in patients with PPH or severe pulmonary fibrosis with associated pulmonary hypertension, the right side is the preferred transplant side. For patients with Eisenmenger’s syndrome, the right side is used to facilitate closure of the coexisting atrial or ventricular septal defect. A patent ductus arteriosus can be repaired in association with transplantation to either side.
Exposure
A generous posterolateral thoracotomy through the fifth interspace or bed of the excised fifth rib is the preferred approach. However, an anterolateral incision through the fourth interspace with division of the medial fourth costal cartilage provides excellent exposure as well. This muscle-sparing approach has been described by Pochettino and Bavaria and has been used successfully in the authors’ program as well.93 For right-sided transplants for which CPB is anticipated, cannulation of the ascending aorta is facilitated by the use of a fourth interspace incision. Median sternotomy can be used for right-sided transplants, especially if associated cardiac repair dictates an anterior approach permitting access to the left side of the heart. The ascending aorta and right atrium can be cannulated easily through the right chest. The cannulas are positioned in the anterior aspect of the incision and remain well out of the operative field throughout the procedure. Through a left posterolateral thoracotomy, the proximal left pulmonary artery and descending aorta can be adequately cannulated.
Recipient Pneumonectomy
After adequate exposure of the pleural space, pleural adhesions are divided. Adhesions can be extensive in patients with fibrotic or septic lung disease but ordinarily are absent in patients with emphysema and PPH. Extreme care is taken not to injure the phrenic and recurrent laryngeal nerves. The inferior ligament is divided. The pulmonary veins and main pulmonary artery are encircled outside the pericardium. During this dissection, the need for CPB is determined. Ventilation of the contralateral lung and occlusion of the ipsilateral pulmonary artery determine whether the contralateral native lung will provide adequate gas exchange and hemodynamics to tolerate pneumonectomy and implantation without CPB. Assessment of right ventricular contractility with the transesophageal echo probe is especially useful at this point.
Easily accessible upper lobe pulmonary artery branches are ligated and divided. This increases the length of pulmonary artery available for subsequent pulmonary artery anastomosis. It also decreases the caliber of the pulmonary artery to match the donor’s size to the recipient’s pulmonary artery, particularly when significant pulmonary hypertension is present. Furthermore, having a ligated recipient upper lobe branch helps
with proper orientation of the donor and recipient pulmonary arteries. The pulmonary artery just distal to this branch is stapled proximally after ensuring that the pulmonary artery catheter is not included in the staple line. A distal pulmonary artery clamp is placed, the vessel is divided, and its distal aspect is ligated to minimize backbleeding, which can be torrential if vigorous bronchial circulation is present.
with proper orientation of the donor and recipient pulmonary arteries. The pulmonary artery just distal to this branch is stapled proximally after ensuring that the pulmonary artery catheter is not included in the staple line. A distal pulmonary artery clamp is placed, the vessel is divided, and its distal aspect is ligated to minimize backbleeding, which can be torrential if vigorous bronchial circulation is present.
The pulmonary veins are divided between staple lines or between silk ligatures placed on each venous branch at the hilum. This latter option increases the size of the subsequent left atrial cuff. Pulmonary artery division is often made easier after division of the superior pulmonary vein.
Peribronchial nodal tissue is divided, and bronchial arterial vessels are secured with ligatures. The bronchus is transected just proximal to the upper lobe origin, and the lung is excised (Fig. 98-6). The recipient bronchus is then trimmed back up into the mediastinum, taking care to avoid any devascularization of the recipient bronchus at the site of anastomosis. The pulmonary artery stump is grasped with a clamp and retracted medially to provide access for the bronchial anastomosis. Similarly, the vein stumps are grasped with clamps, and the pericardium around the vein stumps is opened widely and retracted medially in preparation for the implantation.