INDICATIONS/CONTRAINDICATIONS
Indications
Every potential organ donor should be considered as a potential lung donor. The ideal lung donor criteria as defined in the early years of lung transplantation are listed in Table 1.1. The perfect lung donor matching all these criteria has become very rare in Western Europe because of the constant change in donor profile with a rising proportion of older donors becoming brain death from cerebrovascular disease. The scientific evidence to rely on these strict donor criteria was extensively reviewed by a panel of the Pulmonary Council of the International Society for Heart and Lung Transplantation and found to be very low. Recommendations were made to relax the acceptance criteria. The lung yield from all available donors, therefore, varies between countries and between lung transplant centers within the same country from 10% to nearly 50%. This percentage depends on (1) the expertise of the local donor team with active donor management; (2) the confidence by the recipient team to relax the preset lung donor criteria accepting pulmonary grafts from extended criteria donors and from donors after cardiocirculatory death; and (3) the willingness of the lung retrieval team to travel to the donor hospital to verify whether the initial information regarding lung oxygenation capacity, appearance on chest x-ray, and bronchoscopy findings provided at the time of organ offer, truly reflects lung quality witnessed at final evaluation in the donor after endotracheal suctioning and maximal alveolar recruitment.
Contraindications
As stated above, many potential lung donors will present with one of the more parameters that do not match the ideal criteria listed in Table 1.1. Many of these should be considered as relative contraindications as good immediate outcome is often possible. Nevertheless donors with older age, significant smoking history, inferior oxygenation, and radiographic infiltrates are considered to carry potential risk factors for the onset of chronic allograft dysfunction resulting in impaired long-term survival although strong evidence is missing in published literature. Some donor factors, however, should be considered as absolute contraindications for lung use such as pneumonia or sepsis, significant underlying parenchymal or vascular lung disease, history of recent malignancy (except skin cancer and some brain tumors), ABO incompatibility, and seropositivity for human immunodeficiency virus or hepatitis B or C virus in case of seronegative recipients.
TABLE 1.1 Ideal Lung Donor Selection Criteria

 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
 We prefer to send an experienced lung surgeon to the donor hospital as the decision process whether or not to accept the lung is as important as proper lung preservation and excision.
We prefer to send an experienced lung surgeon to the donor hospital as the decision process whether or not to accept the lung is as important as proper lung preservation and excision.
 Before leaving, the surgeon in charge should check with the scrub nurse and donor coordinator whether all surgical instruments and preservation solutions are available.
Before leaving, the surgeon in charge should check with the scrub nurse and donor coordinator whether all surgical instruments and preservation solutions are available.
 Upon arrival in the donor hospital, the recipient team is quickly informed on the estimated timing of cross clamp in the donor as unexpected delays in transport or start of donor operation may have occurred.
Upon arrival in the donor hospital, the recipient team is quickly informed on the estimated timing of cross clamp in the donor as unexpected delays in transport or start of donor operation may have occurred.
 An advice that is greatly appreciated by younger colleagues when joining for their first lung retrieval is to take off their socks before entering the operating room. Otherwise they risk returning home with a soaked pair after organ flush and additional topical cooling!
An advice that is greatly appreciated by younger colleagues when joining for their first lung retrieval is to take off their socks before entering the operating room. Otherwise they risk returning home with a soaked pair after organ flush and additional topical cooling!
 It is important to introduce the lung team to all members of the local donor team including scrub nurse and anesthesiologist and to retrieval surgeons from other transplant centers.
It is important to introduce the lung team to all members of the local donor team including scrub nurse and anesthesiologist and to retrieval surgeons from other transplant centers.
 In case of simultaneous heart procurement, a plan is discussed and agreed in advance with the heart surgeon on the surgical steps to be taken. Some may not be familiar with combined heart and lung retrieval!
In case of simultaneous heart procurement, a plan is discussed and agreed in advance with the heart surgeon on the surgical steps to be taken. Some may not be familiar with combined heart and lung retrieval!
 It is the surgeon’s responsibility to check all relevant donor medical history, to verify the blood group, and to check whether the death certificate was completed according to state legislation.
It is the surgeon’s responsibility to check all relevant donor medical history, to verify the blood group, and to check whether the death certificate was completed according to state legislation.
 In case the donor is not yet fully installed on the operating table, we prefer to have both arms next to the body to get more working space for both abdominal and thoracic teams. The shoulders are lifted up with towels and the head is extended backwards to have the neck free for better exposure to the trachea.
In case the donor is not yet fully installed on the operating table, we prefer to have both arms next to the body to get more working space for both abdominal and thoracic teams. The shoulders are lifted up with towels and the head is extended backwards to have the neck free for better exposure to the trachea.
 A broad-spectrum antibiotic and 1 g methylprednisolone is administered IV to the donor if not done already.
A broad-spectrum antibiotic and 1 g methylprednisolone is administered IV to the donor if not done already.
 We routinely ask the anesthesiologist in charge of the donor to switch the gas mixture on the ventilator to 100% oxygen and to increase positive end-expiratory pressure to 5 cm H2O if needed. Ventilatory parameters are checked paying attention to the tidal volume and peak airway pressure so to have an idea on pulmonary compliance. A new arterial blood gas sample can be taken after 5 minutes although we prefer to wait for intrathoracic pulmonary vein blood sampling.
We routinely ask the anesthesiologist in charge of the donor to switch the gas mixture on the ventilator to 100% oxygen and to increase positive end-expiratory pressure to 5 cm H2O if needed. Ventilatory parameters are checked paying attention to the tidal volume and peak airway pressure so to have an idea on pulmonary compliance. A new arterial blood gas sample can be taken after 5 minutes although we prefer to wait for intrathoracic pulmonary vein blood sampling.
 First a tracheal aspirate for culture is taken through the endotracheal tube followed by a quick flexible bronchoscopy whenever possible to verify the correct position of the endotracheal tube and to appreciate the amount and color of airway secretions, presence of blood or stomach content, and degree of mucosal inflammation. After cleaning the airways, we routinely perform a bronchoalveolar lavage with 2 × 50 mL of saline solution. The returned fluid is aspirated for culture and cellular and biomolecular analysis. Purulent secretions persistently bubbling up into the central airways are a bad sign indicative for the presence of pneumonia.
First a tracheal aspirate for culture is taken through the endotracheal tube followed by a quick flexible bronchoscopy whenever possible to verify the correct position of the endotracheal tube and to appreciate the amount and color of airway secretions, presence of blood or stomach content, and degree of mucosal inflammation. After cleaning the airways, we routinely perform a bronchoalveolar lavage with 2 × 50 mL of saline solution. The returned fluid is aspirated for culture and cellular and biomolecular analysis. Purulent secretions persistently bubbling up into the central airways are a bad sign indicative for the presence of pneumonia.
 SURGERY
SURGERY
 A standard median sternotomy is performed usually in combination with median laparotomy in case abdominal organ retrieval is scheduled. In case sternotomy is left to be done by our own team (often the chest cavity is already opened by the abdominal team), we prefer to split the sternum in apnea in order not to damage lung parenchyma protruding into the retrosternal plane in a fully ventilated donor.
A standard median sternotomy is performed usually in combination with median laparotomy in case abdominal organ retrieval is scheduled. In case sternotomy is left to be done by our own team (often the chest cavity is already opened by the abdominal team), we prefer to split the sternum in apnea in order not to damage lung parenchyma protruding into the retrosternal plane in a fully ventilated donor.
Lung Inspection and Evaluation
 If not done yet by others, we open the pleural cavity on both sides by incising the mediastinal pleura. Care must be taken not to damage lung parenchyma on its medial aspect when using cautery. At this moment, we do not yet open the pericardium in order not to destabilize the heart too much when inspecting the lungs.
If not done yet by others, we open the pleural cavity on both sides by incising the mediastinal pleura. Care must be taken not to damage lung parenchyma on its medial aspect when using cautery. At this moment, we do not yet open the pericardium in order not to destabilize the heart too much when inspecting the lungs.
 Transection of the diaphragmatic muscles is a tremendous help to gain access with easier exposure of the lower lobes.
Transection of the diaphragmatic muscles is a tremendous help to gain access with easier exposure of the lower lobes.
 The first step is to take a blood sample with a heparinized syringe by puncturing the extrapericardial part of the lower or middle lobe vein on both sides to assess oxygenation capacity (partial oxygen pressure in pulmonary vein = PpvO2) of left and right lungs individually.
The first step is to take a blood sample with a heparinized syringe by puncturing the extrapericardial part of the lower or middle lobe vein on both sides to assess oxygenation capacity (partial oxygen pressure in pulmonary vein = PpvO2) of left and right lungs individually.
 The next step is to inspect the lungs for abnormalities that may preclude safe transplantation. We, therefore, ask the anesthesiologist to disconnect the endotracheal tube from the ventilator after initial preoxygenation as described above. First, it offers the possibility to observe the elasticity of both lungs (“collapse” test). If the lungs do not collapse instantly or symmetrically, this may be indicative of retained secretions or presence of interstitial lung pathology (edema, hemorrhage, pneumonia, emphysema). Deflated lungs are easier to be eviscerated from the pleural cavity without too much cardiac compression. We then quickly palpate both lungs looking for abnormal findings (nodules, blebs, adhesions) that may need further attention during and after retrieval. Significant structural abnormalities may finally exclude the lungs for transplantation.
The next step is to inspect the lungs for abnormalities that may preclude safe transplantation. We, therefore, ask the anesthesiologist to disconnect the endotracheal tube from the ventilator after initial preoxygenation as described above. First, it offers the possibility to observe the elasticity of both lungs (“collapse” test). If the lungs do not collapse instantly or symmetrically, this may be indicative of retained secretions or presence of interstitial lung pathology (edema, hemorrhage, pneumonia, emphysema). Deflated lungs are easier to be eviscerated from the pleural cavity without too much cardiac compression. We then quickly palpate both lungs looking for abnormal findings (nodules, blebs, adhesions) that may need further attention during and after retrieval. Significant structural abnormalities may finally exclude the lungs for transplantation.
 The anesthesiologist is then asked to manually reinflate both lungs with 50% oxygen to a sustained 30 cm H2O pressure for recruitment of all atelectactic lung segments helped with gentle massage by the surgeon (Fig. 1.1). This maneuvre is also very indicative of lung compliance.
The anesthesiologist is then asked to manually reinflate both lungs with 50% oxygen to a sustained 30 cm H2O pressure for recruitment of all atelectactic lung segments helped with gentle massage by the surgeon (Fig. 1.1). This maneuvre is also very indicative of lung compliance.
 Once the blood gas results have returned and PpvO2 values fit with macroscopic findings, the recipient team is called to inform that lungs fulfill quality requirements and what the estimated cross-clamp time will be. Depending on the transport time and estimated operative time needed to explant the native lungs, it is now about the moment to call the recipient to the operating room and to prepare him for induction of anesthesia.
Once the blood gas results have returned and PpvO2 values fit with macroscopic findings, the recipient team is called to inform that lungs fulfill quality requirements and what the estimated cross-clamp time will be. Depending on the transport time and estimated operative time needed to explant the native lungs, it is now about the moment to call the recipient to the operating room and to prepare him for induction of anesthesia.
 In case of discrepancy between healthy looking lungs and low oxygenation capacity (PpvO2/FiO2 <300 mm Hg), blood gas analysis should be repeated to verify.
In case of discrepancy between healthy looking lungs and low oxygenation capacity (PpvO2/FiO2 <300 mm Hg), blood gas analysis should be repeated to verify.
 If any doubt about lung performance and quality, lungs can always be explanted for further testing during ex vivo perfusion in the donor hospital or in the recipient hospital after being transported back on ice in case the expertise and equipment is available.
If any doubt about lung performance and quality, lungs can always be explanted for further testing during ex vivo perfusion in the donor hospital or in the recipient hospital after being transported back on ice in case the expertise and equipment is available.
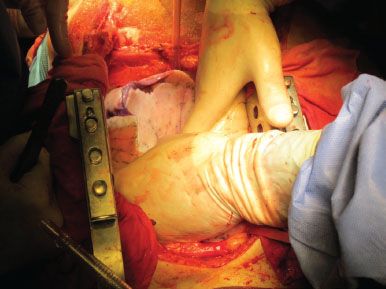
Figure 1.1 All atelectatic parenchymal zones are recruited by pressure ventilation and gentle massage.
Lung Dissection
 If not previously done by others, the next step is to open the pericardium and to suspend both edges to the skin with heavy stitches.
If not previously done by others, the next step is to open the pericardium and to suspend both edges to the skin with heavy stitches.
 In case the heart is retrieved for separate cardiac transplantation, much of the dissection is usually carried out by the heart surgeon. The ascending aorta is freed from the main pulmonary artery and encircled with a tape. The superior vena cava is mobilized from its pericardial attachments and encircled with a heavy ligature distal to both innominate veins. We do not favor to dissect or ligate the azygos vein as this maneuvre may cause bleeding or result in inadvertent ligation of the upper lobe branch of the right pulmonary artery. We have witnessed lobar infarction when arterial transection was not recognized or ignored at implantation. The intrapericardial inferior vena cava is mobilized from its pericardial attachments to facilitate adequate clamping. Passing a ligature is not needed as many abdominal teams like to vent the liver into the pericardium.
In case the heart is retrieved for separate cardiac transplantation, much of the dissection is usually carried out by the heart surgeon. The ascending aorta is freed from the main pulmonary artery and encircled with a tape. The superior vena cava is mobilized from its pericardial attachments and encircled with a heavy ligature distal to both innominate veins. We do not favor to dissect or ligate the azygos vein as this maneuvre may cause bleeding or result in inadvertent ligation of the upper lobe branch of the right pulmonary artery. We have witnessed lobar infarction when arterial transection was not recognized or ignored at implantation. The intrapericardial inferior vena cava is mobilized from its pericardial attachments to facilitate adequate clamping. Passing a ligature is not needed as many abdominal teams like to vent the liver into the pericardium.
 A horizontal mattress suture is then placed in the anterior midportion of the ascending aorta to secure the cardioplegia cannula once it is inserted.
A horizontal mattress suture is then placed in the anterior midportion of the ascending aorta to secure the cardioplegia cannula once it is inserted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


