INDICATIONS
Chest wall reconstruction is indicated in widely different clinical situations, such as resection of tumors, infected or irradiated wounds, congenital deformities, and posttraumatic injuries. In this section we will analyze the various technical options for reconstruction in the oncologic setting.
Chest wall resection and reconstruction for neoplastic disease can be carried out in three different clinical circumstances:
1. primary chest wall tumors;
2. chest wall metastasis or direct infiltration from other malignancies;
3. direct invasion from non-small cell lung cancer (NSCLC).
Such classification is necessary because extent of resection is actually different according to indications: It usually involves some ribs, in the treatment of NSCLC; it often entails a large excision of soft tissues and bony thorax for primary chest wall tumors; it is widely variable in the other possible conditions. Reconstruction may be delegated only to the thoracic surgeon, but in the case of extensive skin and soft tissue resection, repair should include teamwork with plastic surgeons. A multidisciplinary approach is often required.
Primary Chest Wall Tumors
The majority of benign chest wall tumors requiring a thoracectomy arises from the bony thorax (osteochondroma, chondroma), followed by soft tissue tumors (fibrous dysplasia, desmoid tumors, etc.) and by tumors of neural origin (neurofibroma, neurilemmoma). These lesions are often, but not always, slow-growing tumors and are usually asymptomatic, rarely being painful, as in case of osteochondroma or fibrous dysplasia when complicated by pathologic fractures. Chondroma is another common benign tumor, which can easily create problems of differential diagnosis with the corresponding well-differentiated malignant tumor.
Primary malignant chest wall tumors are mainly represented by sarcomas. The most common is chondrosarcoma, a chemo- and radioresistant tumor, its prognosis is related to its grade, dimension, and width of resection. Other malignancies are Ewing’s sarcoma, osteosarcoma, synovial sarcoma, fibrous histiocytoma, plasmacytoma, and a variety of soft tissue sarcomas. Ewing’s sarcoma and plasmacytoma are responsive to chemotherapy. Different biology can grade these tumors from indolent to rapidly aggressive and occasionally related to latent or manifest systemic disease. Multifocal tumor is a possible entity that heavily limits the surgical indication.
Metastatic Chest Wall Tumors
Bloodstream metastases to the chest wall infrequently occur from epithelial tumors such as carcinomas of the thyroid, breast, and kidney, but also from other primaries. Palliation is generally obtained by radiation therapy and resection is rarely indicated.
Surgery might be considered in selected cases: (a) As part of a multidisciplinary treatment program (e.g., differentiated thyroid tumors); (b) as a treatment of a local complication such as radiation or infected wound (occasional in breast cancers); (c) for a bleeding lesion (typical of renal cell carcinoma); (d) for the sole palliation, generally for pain relief (occasional in every histology).
Chest Wall Involvement by Lung Cancer
Peripheral NSCLC sometimes infiltrates the parietal pleura or the chest wall and this condition is defined as T3 tumor; invasion of the vertebral body is defined as T4. In patients with vertebral body involvement surgery is rarely indicated and the related techniques will not be analyzed in this chapter. Surgical indication is disputed in T3N2 disease, but cT3N0–1 can undergo en-bloc chest wall and lung resection with satisfactory oncologic outcome. Many experiences encourage the role of surgery but there are some peculiar aspects that define the indication to surgery as part of a possible multimodality treatment. Prognostic factors are multiple and mainly dependent on complete resection, N status and depth of infiltration; the latter is also a key factor to assess the correct technique. Currently there are some uncertainties on the extent of resection. When the tumor clearly infiltrates ribs and/or soft tissues, the only radical operation is the concomitant chest wall and lung en-bloc resection. If the parietal pleura is marginally infiltrated, extrapleural lobectomy without chest wall resection could be performed, provided that the extrapleural plane is easily achieved and the outer surface of the detached parietal pleura is left absolutely intact (however, only the final pathologic examination can definitely rule out the possible full-thickness parietal pleural involvement). It is important to interrupt the extrapleural dissection in case of resistance to the maneuver. If the procedure goes smoothly, a full-thickness chest wall resection could be an overtreatment because the tumor can be fixed to the parietal pleura only by inflammatory adhesions, or be confined just to the pleural plane. The decision must be made intraoperatively and requires an experienced surgeon.
If the parietal pleura is invaded, many authors deem extrapleural lobectomy a not surely radical operation because of the higher probability of local recurrence. In fact, although data are not definitive, in T3 tumors, chest wall and lung en-bloc resection should lead to better long-term outcome than extrapleural lobectomy. Anyhow, concomitant chest wall and lung resection is unquestionably required if the patient complains chest pain or if parietal pleura infiltration is not minimal.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Evaluation of the Patient
Functional Assessment
Analysis of preoperative functional studies is beyond the scope of this chapter. Synthetically, we should consider adequate for a major chest wall resection the same functional respiratory, metabolic, and cardiovascular parameters used to judge a patient eligible for a pulmonary lobectomy. If a large thoracectomy en-bloc with a major pulmonary resection has been scheduled, the patient should be functionally fit for pneumonectomy.
Clinical Data of the Patient
Chest wall resection should be undertaken after a meticulous treatment plan resulting from deep knowledge of the disease and adequate assessment of the patient. Clinical history and physical examination are fundamental both for correct diagnosis and therapeutic plan.
Multiple factors influence the technique of resection and reconstruction of the chest wall: Some of them are related to the patient and to his clinical history, others are linked to the disease.
The most important patient-related factors to be considered are: Comorbidities, performance status, symptoms, lifestyle, occupation, age, previous radiation therapy, previous surgery or chemotherapy, body habitus, infection, skeletal muscle function, body mass, and nourishment. Chest pain should be carefully investigated because it is the most important sign of local invasion. The growth rate of the tumor, when detectable, is a critical prognostic factor. Thorough physical examination is essential for surgical planning: The characteristics of the mass, its relationship with both the superficial and deep layers, and any local sign of infection must be carefully examined.
Tumor biology, prognosis, and possible multimodal treatment planning represent the most important disease-related factors, influencing the presumed extent of resection and the kind of reconstruction.
Instrumental Evaluation of the Disease
Imaging
Contrast-enhanced computed tomography (CT) is the imaging test of choice to define size, localization, radiodensity, shape, contour, margins, boundaries, homogeneity/heterogeneity, calcifications, necrosis, vascularity, patterns of contrast enhancement, and distant metastasis; cleavage planes and possible infiltration into adjacent structures may be not definitively determined in all patients.
Magnetic resonance imaging (MRI) is to be considered complementary and not alternative to CT scan. It is recommended in selected cases to evaluate soft tissue planes and to better assess neural, spinal, and vascular involvement.
Biopsy
Even though advances in imaging techniques can make biopsy unnecessary in very selected cases, tissue diagnosis is essential for a correct treatment strategy. In fact, only when the benign or malignant nature of the chest wall tumor has been established, surgical planning can be correctly drawn up. Furthermore, some diseases such as chest wall metastases, Ewing’s sarcoma, and plasmacytomas require chemotherapy and surgery should be only considered as part of a multimodal approach.
Biopsy options include fine-needle aspiration, core-needle biopsy, incisional biopsy, and excisional biopsy. Fine-needle aspiration has a poor diagnostic yield in primary chest wall tumors and should be performed only if chest wall invasion from lung cancer or from other malignancies is suspected. Conversely, the very high diagnostic accuracy of core-needle biopsy makes incisional biopsy rarely needed in the diagnosis of primary tumors. Excisional biopsy is mandatory in case of a chondromatous lesion but can be a reasonable alternative to minimal biopsies also for other tumors, providing that the resulting chest wall defect is small. In conclusion, the choice between the different options for biopsy must be essentially individualized on the basis of the features of the lesion.
Nuclear Imaging Tests
Both 18FDG-positron emission tomography and bone scan may be useful in selected cases to assess the extent of the disease.
 SURGERY
SURGERY
The correct approach comes from the analysis of three different points:
a. Resection
b. Restoration of skeletal stability
c. Soft tissue coverage
Resection
The extent of resection varies depending upon the indications.
Benign chest wall tumors: The correct treatment is tumor removal with clear margin. As a rule, resection should not be extended to the skin and the adjacent musculature if not clearly required, but care must be taken to avoid an incomplete resection. In selected cases a wider excision is recommended for the risk of an undiagnosed malignant tumor (chondrosarcoma), or for the possible high local recurrence rate (Desmoid tumors).
Primary malignant chest wall tumors: Wide en-bloc resection is the key for a successful management. It is generally defined as wide en-bloc resection of a tumor excision with 4-cm free resection margins, including the involved skin and soft tissues, ribs and/or sternum, and any other structure invaded by the disease. If a previous surgical biopsy has been done, en-bloc resection of the entire biopsy site must be performed, to avoid the high risk of tumor seeding during the procedure. Radical surgery is often the only real therapeutic chance and the extent of resection should not be limited by anticipated difficulties in reconstruction.
Chest wall direct invasion by lung cancer: Chest wall and lung en-bloc resection is the standard of care. There is no unanimous agreement about the security margin: Theoretically one rib above and below the macroscopic tumor should be recommended with a lateral margin of 3 to 4 cm. Some authors deem 1 cm of free margin in all directions sufficient to balance the surgery-related morbidity, being complete resection the goal to be achieved.1 We think that the security margin must be possibly wide: A minimum resection margin, even if microscopically negative, should be categorized as a compromise solution, to be reserved for those patients in whom the other technical solutions would result in an excessive surgical trauma. In this context, the most common situation is chest wall invasion near the paravertebral sulcus without direct spine involvement, where a minimum histologically negative resection margin could be considered acceptable, making a balanced assessment between risk of local recurrence and trauma related to the vertebral bodies resection.
Chest wall metastasis or direct invasion from other malignancies: A minimum resection margin is generally considered satisfactory. Surgery is rarely indicated, if any, always in the context of a multimodal treatment. If resection is required for a radiation wound or for an ulcerated, infected tumor, wide resection should be performed: Despite extensive resection and apparent removal of any residual infected tissue, the use of synthetic prosthetic material is contraindicated and the resulting dead space must be obliterated by well-vascularized flaps, preferably by a pedicled omental flap.
Technique
Resection of primary or secondary chest wall tumors must be performed en-bloc with the adjacent involved tissues, to avoid tumor seeding. Only few tips and tricks may be provided: The procedure is not technically demanding but a precise method is required. When dealing with lung cancer infiltrating the chest wall, thoracectomy should be performed first, going ahead with en-bloc lobectomy only when the involved chest wall has been freed into the pleural cavity. The chest should be approached well distant from the involved area, to properly assess the local extent of disease, without risks of tumor seeding. Chest wall resection should start with the easier side to expose (i.e., from back to front, for anterior thoracectomies; from front to back, for posterior resection; from below to above, for resection of the first five ribs and vice versa for lower rib resections). In selected lung cancer patients requiring extended thoracectomy, a preliminary atypical lung resection with linear staplers may be useful, provided that a macroscopically free resection margin has been achieved. Subsequently, thoracectomy en-bloc with sublobar resection is carried out. Completion lobectomy and mediastinal lymph node dissection can be performed at the end of the demolitive phase, without any technical obstacles. This surgical strategy facilitates both chest wall and lung resection; in fact, lung adhesion to the chest wall may preclude adequate exposure of the opposite side of the ribs to be removed and a large segment of resected chest wall, dropped into the pleural cavity, can hinder hilar dissection and prevent the lung to be moved inside the chest. However, in most cases requiring an extended thoracectomy, a wide wedge resection is not feasible with a safe margin and the above described technique may be contraindicated.
In posterior thoracectomies, resection of the vertebral transverse process must be carried out with caution. In fact, control of bleeding near the intervertebral foramen is extremely delicate due to the adjacent spinal cord; moreover, the dura mater can be torn during paravertebral sulcus dissection, with possible cerebrospinal fluid leak. For such reasons, the vertebral transverse process should be resected only if the head of the rib is dorsally infiltrated by the tumor; in the other conditions, if necessary, the ribs can be completely disarticulated with a precise technique. Ribs are strongly connected to the spine by a peculiar kind of articulation. Rib head articulates with the body of the thoracic vertebrae and the rib tubercle with the transverse process, with a relatively long overlapping of the bone segments. This type of articulation is categorized as arthrodial joint and is characterized by tight joint capsules, strengthened by multiple and tough ligaments. To achieve complete rib disarticulation, anterior costal interruption is performed first, to allow a higher mobility of the posterior segment of the chest wall to be resected. The erector spinae muscles are then incised to expose the costotransverse joint. The tubercular ligament is cauterized and a curved heavy periosteal elevator is inserted between the transverse process and the rib (Fig. 16.1). To release the neck of the rib it is necessary to divide the costotransverse ligament, which is generally very strong, so much so that rib neck fracture may be easily produced. The cautious use of a light hammer can be helpful to facilitate insertion of the periosteal elevator. The rib neck is dislocated anteriorly by the periosteal elevator, wedged forward with a progressive lever action, until the costal head has been detached from the vertebral body; at the end of the procedure the residual costovertebral ligaments are divided by scissors.
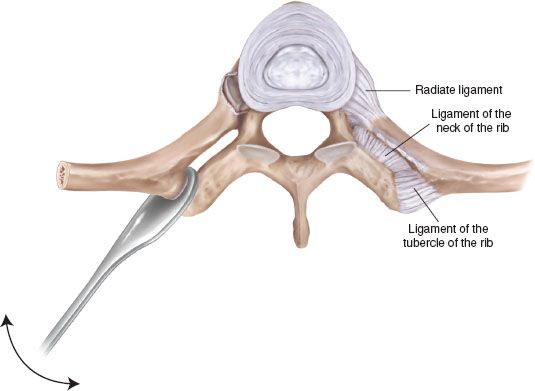
Figure 16.1 The periosteal elevator is wedged forward between rib tubercular facet and vertebral transverse process. Vigorous, active pendulum movement (arrow) achieves disarticulation without fracture.
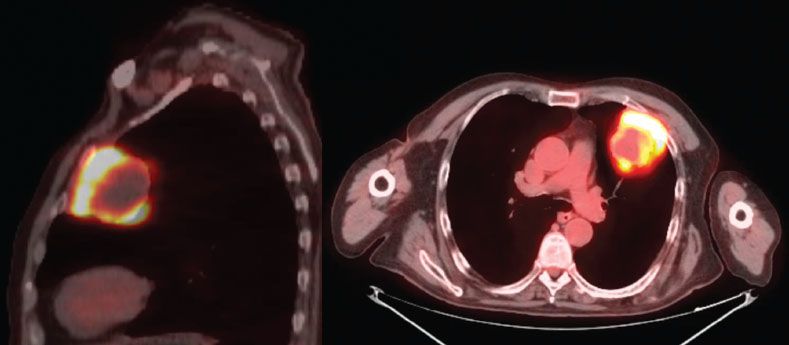
Figure 16.2 PET-CT scan of a cT3 left upper lobe lung cancer, suitable for double-step anterior resection and reconstruction.
For lung cancer invading very anteriorly the chest wall and requiring en-bloc thoracectomy with division of costal cartilages, an anterolateral thoracotomy is the most intuitive choice because surgical dissection of the costochondral joints is hampered by lung infiltration, if approached posteriorly (Fig. 16.2). On the other hand an anterior approach may have two drawbacks: (1) It does not allow the ideal surgical exposure of the chest wall, posteriorly to the midaxillary line; (2) the resected thoracic wall segment, released within the pleural cavity, is cumbersome and can hamper hilar dissection. In such circumstance, we found advantageous a preliminary short longitudinal parasternal incision to cut the invaded costal cartilages close to the sternum, easily achieving the appropriate resection margin. Before wound closure, a variable number of heavy nonabsorbable stitches are placed on the remaining healthy tissues and/or through the sternum, to medially anchor the future prosthesis: The needles are removed and the stitches knotted distally and temporarily abandoned inside the chest. Then a posterolateral thoracotomy is performed to conclude the en-bloc pulmonary resection facing the easier lateral side of the thoracectomy. The reconstructive phase is facilitated by fixing the prosthesis to the far anterior border of the defect, collecting the anterior stitches previously placed, which are secured to the prosthetic mesh by free needle (Fig. 16.3).
Restoration of Skeletal Stability
The reconstructive technique should ensure early return to normal breathing, protection of intrathoracic organs, restoration of physiologic volume of the rib cage, and satisfactory cosmetic result.
Return to efficient ventilation and protection of intrathoracic viscera are the fundamental targets. A multidisciplinary team, including plastic surgeons, is recommended if an extensive soft tissue and skin resection has been scheduled: It is worth underlining that correct surgical planning constantly requires soft tissue coverage and skin closure, while rigid stabilization of the bony thorax is not always necessary and represents a controversial issue. Reconstruction of the bony thorax is unnecessary for small defects not overlying cardiac structures, which do not significantly impair breathing. In fact, soft tissues reconstruction may provide normal respiratory mechanics in patients with a small chest wall defect and good baseline lung function. Large full-thickness defects not adequately stabilized may act as a sort of traumatic flail chest. However, the two conditions are not exactly comparable: Paradoxical movement of the chest wall after multiple ribs resection is usually not a life-threatening condition as severe traumatic flail chest could be. Probably for these reasons, some surgeons do not usually repair the bony defect, thus underestimating the problem of paradoxical breathing.2 We do not agree with this behavior, although there is no conclusive evidence to support the necessity for bony reconstruction of a large chest wall defect. First of all, an inadequate bony reconstruction after wide thoracectomy may have serious pathophysiologic consequences, affecting both postoperative course and pulmonary status of the patient. Secondly, progress currently achieved in the field of chest wall prostheses allows an easy and safe stabilization of the bony thorax, with minimal morbidity related to the reconstructive procedure. A significant paradox impairs ventilatory mechanics, weakens cough effectiveness, causes mucus retention, increases the risk of pneumonia, and often leads to prolonged postoperative mechanical ventilation, which in itself increases the risk of infection. Respiratory failure is the possible final outcome of these pathophysiologic events. The high incidence of respiratory complications reported after chest wall resection has been correlated to the residual paradoxical movement, resulting from an inadequate reconstruction. In fact, a lower incidence of respiratory complications has been reported by those authors who systematically use skeletal stabilization for large defects.3 For such reasons we believe that any chest wall defect that has the potential for paradox, requires restoration of the skeletal stability. Regardless of the adequacy of soft tissue coverage, prevention of the paradoxical chest wall movements should represent a fundamental goal in surgical planning.
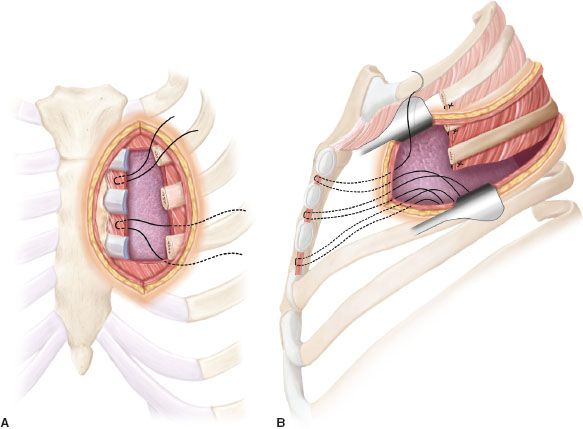
Figure 16.3 A: Preliminary limited longitudinal left parasternal incision: The sternocostal joints of the second, third, and fourth ribs are interrupted and nonabsorbable stitches are placed on the peristernal tissues and temporarily abandoned inside the chest cavity. B: Posterolateral thoracotomy allows to easily complete the posterior section of the involved ribs and the pulmonary resection. Chest wall reconstruction is facilitated by fixing the prosthesis to the far parasternal border of the defect, collecting the anterior stitches previously placed.
Size and location of the defect are the two interdependent factors mainly influencing the likelihood of occurrence and the entity of a postoperative paradox.
Size of the defect. Number, length of rib resection and width of skin soft tissues excision are to be taken into account. There is not a definite threshold that, per se, makes rigid stabilization of a chest wall defect mandatory, even though, roughly, it would be preferable to reconstruct every large bone defect. It has been reported that the >5-cm resection of two consecutive ribs should require rigid stabilization but actually, even a larger defect may be left unreconstructed, thus proving that location more than size of the defect is the basic factor in the decision-making process.
Location of the defect. To guide the decision whether bony chest wall reconstruction is necessary or not, the hemithorax can be topographically divided into “noncritical” and “critical” areas. The latter usually require skeleton reconstruction after full-thickness resection.
Noncritical areas are the apical and the posterior regions; critical areas are the basal, the lateral, and the anterior regions.
The apical region (including first, second, and third ribs) is frequently involved by chest wall resection for the treatment of Pancoast tumors. Resection of the posterior half of the first three ribs does not require bony reconstruction, because the defect lies beneath the scapula. Even if complete resection of the first three ribs is performed, stabilization is not required since the resulting defect works as a thoracoplasty, causing reduction in size of the chest cavity with obliteration of the apical pleural space.
The posterior region is located between the posterior spinal line and the posterior axillary line. Chest wall instability is rarely significant in this area for the following reasons: It is well protected by a thick layer of muscles (latissimus dorsi, trapezius muscle); the supine decubitus of the patient reduces the paradoxical movement of the chest wall. Bony reconstruction is required if the tip of the scapula can fall within the defect and get trapped during movements of the arm (such event must be anticipated if the defect includes the fifth rib). In case of extended soft tissue resection, reconstruction of the posterior thorax is usually achievable by a variety of pedicled flaps.
The lateral region
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


