CHAPTER 16 Lung Cancer Workup and Staging
It was estimated that in 2008 there were approximately 215,020 new cases of lung cancer in the United States,1 and with 161,840 deaths projected for 2008, lung cancer remains the leading cause of cancer-related deaths in both men and women. Of newly diagnosed cases, approximately 80% will be non–small cell lung cancer (NSCLC), and of these, 80% will involve disseminated or locally advanced disease. Unfortunately, only 20% will be in potentially surgically curable patients with early stage disease, where complete resection yields a 5-year survival rate approaching 70%.2
THE STAGING SYSTEM
In 1974, the American Joint Committee for Cancer (AJCC) Staging developed a lung cancer staging system based on TNM descriptors.3 Naruke and coauthors4 devised the original lymph node mapping schema that placed nodes into stations on the basis of clearly defined anatomic boundaries. Used to define the N (nodal) descriptors, it was modified for North America by the American Thoracic Society5 and by Mountain and Dresler.6 The current form of the map is seen in Figure 16-1. In 1986, the AJCC, Union Internationale Contre le Cancer (UICC), and representatives from Japan and Germany proposed an International Staging System (ISS) for lung cancer that grouped patients with similar survival outcomes using anatomic and morphologic criteria.7 This system was originally applied to a database of over 3000 patients from the M. D. Anderson Cancer Center and the Lung Cancer Study Group and verified significant survival differences between stages. The applicability of the ISS was subsequently confirmed by studies from Naruke and colleagues8 and Watanabe and coworkers.9 The version of the ISS adopted in 1997 attempted to further minimize heterogeneity within stages and refine prognostic groupings after reviewing the survival data of 5319 patients.10 The significant changes included dividing stages I and II into A and B subsets and incorporating T3N0M0 tumors into stage IIB. In addition, satellite nodules in the same lobe of the lung as the primary lesion were designated as T4 disease, whereas all other ipsilateral synchronous lesions were designated as M1. The current sixth edition of TNM Classification of Malignant Tumours introduced in 2002 made no alterations to the previous edition with respect to lung cancer.11
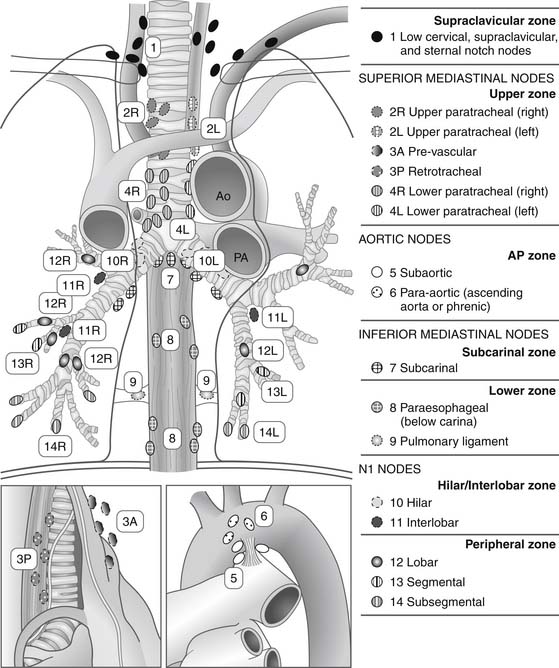
Figure 16–1 Regional lymph node stations for the staging of non–small cell lung cancer. Ao, aorta; PA, pulmonary artery.
(From Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC Lung Cancer Staging Project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2009;4:568.)
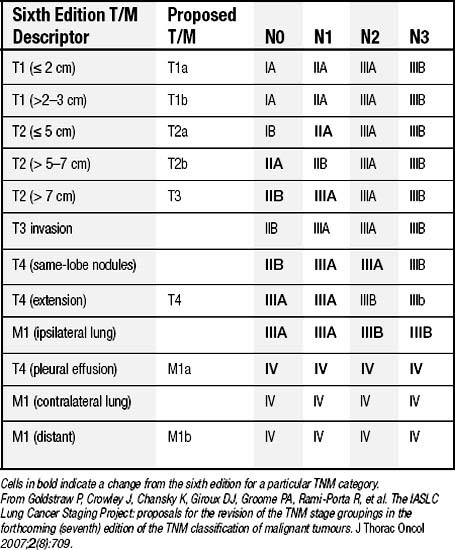
In 1996, the International Association for the Study of Lung Cancer (IASLC) initiated an international staging project to form the basis of the seventh edition of the TNM staging system.12 The goals of the project were to validate the individual T, N, and M descriptors using a larger database made up of medical and surgical patients from a wide geographic distribution.13 Data on 100,869 patients, including 67,725 cases of NSCLC, were submitted to the database, and several changes were proposed.14 The existing N descriptors were validated and not changed.15 For the T descriptors, suggestions were made to make additional size cutoffs to the T1 and T2 tumors and to designate tumors greater than 7 cm in greatest dimension as T3, recognizing the poor prognostic significance of increasing size of the primary tumor (Table 16-1).16 Furthermore, additional tumor nodules in the same lobe as the primary are proposed to be T3, whereas other nodules in the ipsilateral lobes become T4. It was recommended that the M descriptor be divided into M1a (for pleural metastases, malignant effusion, or contralateral pulmonary disease) and M1b (for extrathoracic metastases).17 After incorporating the suggested changes into the data and reanalyzing the TNM subsets, new stage groupings were identified that yield even distributions between stages, particularly between stages IIA and IIB (Table 16-2).
Table 16–1 Proposed Definitions for T, N, and M Descriptors
| T (Primary Tumor) | |
| TX | Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤ 3 cm in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus)∗ |
| T1a | Tumor ≤ 2 cm in greatest dimension |
| T1b | Tumor > 2 cm but ≤ 3 cm in greatest dimension |
| T2 | |
| T2a | Tumor > 3 cm but ≤ 5 cm in greatest dimension |
| T2b | Tumor > 5 cm but ≤ 7 cm in greatest dimension |
| T3 | Tumor > 7 cm or one that directly invades any of the following: chest wall (including superior sulcus tumors), diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium; or tumor in the main bronchus < 2 cm distal to the carina∗ but without involvement of the carina; or associated atelectasis or obstructive pneumonitis of the entire lung or separate tumor nodule(s) in the same lobe |
| T4 | Tumor of any size that invades any of the following: mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina; separate tumor nodule(s) in a different ipsilateral lobe |
| N (Regional Lymph Nodes) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension |
| N2 | Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s) |
| N3 | Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) |
| M (Distant Metastasis) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Separate tumor nodule(s) in a contralateral lobe; tumor with pleural nodules or malignant pleural (or pericardial) effusion† |
| M1b | Distant metastasis |
∗ The uncommon superficial spreading tumor of any size with its invasive component limited to the bronchial wall, which may extend proximally to the main bronchus, is also classified as T1.
† Most pleural (and pericardial) effusions with lung cancer are due to tumor. In a few patients, however, multiple cytopathologic examinations of pleural (pericardial) fluid are negative for tumor, and the fluid is nonbloody and is not an exudate. Where these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging element and the patient should be classified as T1, T2, T3, or T4.
From Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2(8):709.
DIAGNOSIS AND STAGING
History and Physical Examination
Patients often present for evaluation with a number of studies already performed. However, for several reasons, the history and physical examination remain the most important initial steps in evaluating patients who are suspected of having lung cancer. A detailed history focusing on risk factors such as duration of cigarette smoking, exposure to asbestos and other industrial hazards, a prior history of lung cancer, and the presence of symptoms allow the clinician to assess the probability of the diagnosis of lung cancer. Bach and associates showed that the duration of tobacco smoking, more so than the amount of daily usage, increases an individual’s risk of developing lung cancer.18 Similarly, the risk associated with asbestos increases with the intensity and length of exposure, and together, tobacco use and asbestos exposure have a multiplicative effect. Certain symptoms, such as bone pain, hoarseness, weight loss, and neurologic changes, can indicate the presence of metastatic disease and mandate further investigation.
NONINVASIVE MODALITIES
Chest Radiography
The standard posteroanterior and lateral chest radiographs are the most common initial study in which a suspected lung cancer is identified. Lesions are detected on a chest radiograph performed for symptoms or for unrelated reasons, such as part of a routine health assessment or preoperative clearance. Most lesions are not visible until they are at least 7 to 10 mm in diameter,19 at which time they contain roughly 1 billion cells, representing 30 doublings.20 A high-quality chest radiograph can impart a large amount of information and should be reviewed carefully. It localizes the site of suspect lesions (central or peripheral) and the local extent and effects of disease, showing areas of atelectasis, consolidation, or proximity to the pleural surface. The presence of a pleural effusion indicative of a T4 tumor can be seen, as well as elevation of the hemidiaphragm in the event of phrenic nerve involvement. Advanced disease may be identified in the case of rib destruction from bone metastases or synchronous lesions in the pulmonary parenchyma. Hilar and mediastinal lymph node metastasis is more difficult to identify, unless there is substantial enlargement.
Sputum Cytology
Cytologic analysis of sputum for malignant cells is a simple diagnostic technique, although it is being used less often in North America than in the past because bronchoscopy and percutaneous needle biopsy are used more now, and because of the decrease in the proportion of squamous cell cancers. Samples obtained may be induced by saline nebulization or collected as a 3-day pool of sputum produced from spontaneous coughing in the morning. Induced specimens should be immediately fixed and stained. Pooled samples are preserved in Saccomanno’s solution (50% ethanol and 2% polyethylene glycol), or 70% ethanol until fixation and staining can be performed. Schreiber and McCrory21 performed a meta-analysis of the published data on the diagnostic accuracy of sputum cytology in lung cancer. Most studies included a variety of indications for testing with only a few evaluating the sensitivity and specificity of sputum cytology in patients suspected of having lung cancer. Based on 16 published studies of at least 50 patients each, the overall sensitivity is 66% (range, 42% to 97%) and the overall specificity is 99% (range, 68% to 100%). The false-positive and false-negative rates are 9% and 6%, respectively. When sputum cytology is used for patients suspected of having lung cancer on clinical grounds, the diagnostic yield is higher, with a sensitivity of 87% and a specificity of 90%, as demonstrated by Jay and colleagues.22
A number of authors have shown that the sensitivity of sputum cytology for detecting lung cancer depends on the number of specimens collected per patient. Pilotti and coworkers,23 Liang,24 and Böcking and coworkers25 confirmed that the optimal diagnostic sensitivity requires three specimens per patient. Other factors that influence the diagnostic sensitivity of sputum cytology include tumor location, size, and histologic type. Schreiber and McCrory21 reviewed 17 studies that compared the sensitivity of sputum cytology in central versus peripheral lung lesions. Meta-analysis showed that the overall sensitivity was 71% for central lesions and 49% for peripheral lesions. The most likely explanation is that central tumors have a higher likelihood of shedding tumor cells into expectorated sputum because of their proximal bronchial location. Kato and associates26 found that large tumors (greater than 3 cm), tumors associated with atelectasis or consolidation, and lower lobe location had higher diagnostic yields. In their study, the lowest sensitivity (20%) for sputum cytology occurred with peripheral tumors less than 3 cm in diameter. Histology of the tumor generally has less influence on the diagnostic accuracy of sputum cytology than the other factors mentioned. Squamous carcinomas are, however, more frequently diagnosed by sputum analysis than adenocarcinomas or large cell carcinomas. This is probably related to the fact that squamous tumors are more commonly central in location.
High-Resolution Computed Tomography
In addition to providing information about the primary tumor and the possibility of distant metastases, CT of the chest allows assessment of mediastinal lymph nodes. If not contraindicated, intravenous contrast is useful in distinguishing nodes from vascular structures. The most widely used criterion to define metastatic involvement is a short-axis diameter of 1 cm or greater. Although it is the most effective radiographic method of measuring lymph node enlargement, CT alone is inadequate to reliably predict metastatic nodal disease. In a prospective study of 143 patients with bronchogenic carcinoma in which CT findings were correlated with surgical pathologic staging, McLoud and coauthors27 reported that the sensitivity of CT in predicting mediastinal nodal status was 64%, with a specificity of 62%. Toloza and colleagues28,29 reported results of a meta-analysis of 20 studies with 3438 evaluable patients that showed an overall sensitivity of 57% and specificity of 82%. Therefore, staging of the mediastinum and subsequent therapeutic decisions should not be based solely on the results of the CT in most cases.
Positron Emission Tomography
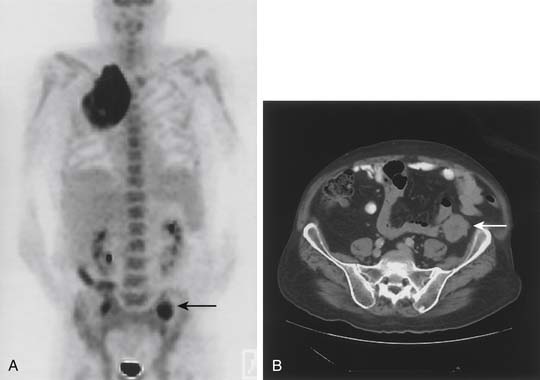
Whole-body PET is a physiologic imaging technique based on the detection of positrons emitted by low-atomic-weight isotopes (carbon, fluorine, oxygen, nitrogen). Fluorodeoxyglucose (FDG) labeled with radioactive fluoride (18F) is a D-glucose analog that is phosphorylated after cellular uptake and accumulates intracellularly, rather than being metabolized. Because lung cancer cells have an increased rate of glycolysis and overexpress the glucose transporter, there is preferential accumulation and visualization of FDG in the primary tumor and in potentially metastatic sites (Fig. 16-2).30 The criterion for an abnormal PET scan is either a standardized uptake value (SUV) of greater than 2.5 or uptake in the lesion that is greater than the background activity of the mediastinum. Currently, the lower limit of resolution of PET is approximately 1.0 to 1.2 cm. In addition, certain non-neoplastic processes (such as inflammatory conditions and infections) can produce false-positive PET findings. Despite these limitations, PET has become an invaluable tool in lung cancer staging.
Several recent studies have shown that PET is superior to CT in staging the mediastinum in lung cancer patients. Pieterman and colleagues31 prospectively compared standard staging approaches to PET for detection of mediastinal lymph node and distant metastases in 102 patients with resectable NSCLC who underwent histologic staging of the mediastinum. The sensitivity and specificity of PET for detection of mediastinal nodal metastases were 91% and 86%, respectively, compared with a sensitivity of 75% and a specificity of 66% for CT. Similarly, meta-analysis of 18 studies by Toloza and coworkers28,29 demonstrated an overall sensitivity of 84% and specificity of 89%. Pieterman and associates31 also showed that combining CT and PET resulted in the highest diagnostic accuracy, with a sensitivity of 94% and a specificity of 86%, supporting the use of both modalities for staging of the mediastinum.
Another significant result in the study by Pieterman and coauthors31 was the observation that PET resulted in a stage different from the one arrived at by the standard methods in 62 of 102 patients, correctly indicating a lower stage in 20 patients and higher-stage disease in 42 patients. More importantly, PET was effective in identifying occult metastatic disease. In 11 patients (11%), PET demonstrated distant metastatic disease that was not detected by the usual staging tests. The sites of metastasis included bone, liver, and adrenal gland.
Within the past 10 years, integrated PET-CT scanners have replaced dedicated PET alone because of improved diagnostic accuracy and anatomic localization of disease.32 Lardinois and colleagues33 compared the diagnostic accuracy of integrated PET-CT with CT alone, PET alone, and visually correlated PET and CT in 50 patients with proved or suspected NSCLC. In 40 patients who underwent histologic confirmation, tumor staging was significantly more accurate with integrated PET-CT than with CT alone (P = .001), PET alone (P <.001), or visual correlation of PET and CT (P = .013). In 37 patients with histologic confirmation of nodal status, integrated PET-CT was more accurate in assessing the mediastinum than PET alone (P = .013). Moreover, unsuspected extrathoracic metastases were discovered in 16% (8 of 49 patients). Similarly, Cerfolio and coauthors compared PET with and without integrated CT in 129 patients with biopsy-proved or suspected NSCLC who subsequently underwent surgical staging.34 PET-CT was a significantly better predictor of stages I and II disease and demonstrated superior accuracy for both T status (70% versus 47%, P = .001) and N status (78% versus 56%, P = .008) compared with PET alone. Moreover, PET-CT was more sensitive and more specific and had a higher positive predictive value for the status of N1 and N2 disease (P < .05). As in prior studies, PET-CT identified 19 (14.7%) patients with M1 disease.
With respect to the evaluation for the presence of bone metastases, PET-CT has proved to be more accurate than a bone scan, with a similar sensitivity and a higher specificity.35–37 In the largest study comparing PET with bone scan, Song and colleagues retrospectively analyzed 1000 newly diagnosed patients, 105 of whom were eventually diagnosed with bone metastases and underwent PET-CT and bone scan.37 PET-CT was more accurate (98.3% versus 95.1%, P < .001), sensitive (94.3% versus 78.1%, P = .001) and specific (98.8% versus 97.4%, P = .006) than bone scan. PET-CT also showed a lower incidence of false positives (1.2% versus 2.9%) and false-negative results (5.7% versus 21.9%) compared with a bone scan. Agreement between PET-CT and bone scan findings was good, with a calculated κ = 0.732. Based on its clear advantages in noninvasive intrathoracic staging, and particularly in the identification of occult extrathoracic disease, PET-CT should be a routine part of pretreatment staging.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree