CHAPTER 17 Lung Cancer
Surgical Treatment
This chapter provides an overview of the surgical treatment for lung cancer. The indications for surgery, types and extent of lung resection, and mediastinal lymphadenectomy are reviewed, with an emphasis on recent published evidence and the new international tumor-node-metastasis (TNM) staging system (seventh edition, 2009). Minimally invasive lung resection (see Chapter 18), tracheal lesions (see Chapter 8), tumor invading the chest wall (see Chapter 20), and multimodality approaches (see Chapter 19) are specifically covered separately.
Lung cancer remains the leading cause of cancer death worldwide, with an estimated 160,390 deaths and 213,380 new cases in the United States in 2007.1 Complete surgical resection is the treatment of choice for patients with early-stage (stages I and II) and selected locally advanced (stage III) non–small cell lung cancer (NSCLC). There are also several situations where surgery may have a role to play: NSCLC with isolated adrenal metastasis, isolated brain metastasis, or highly selected small cell lung cancer (SCLC). Adjuvant chemotherapy has been established as a modality to improve survival in selected resectable NSCLC on the basis of published clinical studies including phase III randomized trials.2 Neoadjuvant therapy (induction chemotherapy or chemoradiotherapy) improves outcome in patients with N2 disease or resectable, locally advanced NSCLC.3
HISTORICAL NOTE
The first lobectomy for lung cancer was described by Davies4 in 1912; the patient died 8 days after resection, with empyema. As water-sealed drainage system and anesthetic techniques advanced, surgical resection for lung cancer became prevalent. In 1933, Graham and Singer5 reported the first successful one-stage pneumonectomy. Since then, the standard operation for lung cancer became pneumonectomy with the technique of individual hilar ligation of the pulmonary vessels and suturing of the bronchus. Around the same time, Churchill and coworkers6 described their experience of lobectomies with hilar dissections. The technique of segmentectomy for lung cancer was described by Overholt and Langer.7 As refinements progressed, more advanced techniques were developed, including sleeve resection by Price-Thomas8 in 1947, carinal resection by Mathey and colleagues9 and Thompson,10 and Pancoast tumor resection by Chardak and MacCallun.11 Grillo and colleagues12 reported on carinal resection with airway reconstruction in 1963. In 1973, Jensik and colleagues13 reported a large series of segmental resections as intentional curative procedures for the treatment of bronchogenic carcinoma. Since the early 1990s, as the system of video endoscopy and endoscopic instruments have evolved, video-assisted thoracoscopic surgery (VATS) for major pulmonary resection has continued to develop worldwide.14
PULMONARY SURGICAL OPERATIONS: INDICATIONS AND TECHNIQUE
Lobectomy
Lobectomy with complete en bloc tumor removal remains the standard surgical procedure in patients with resectable NSCLC. Resections less extensive than lobectomy (i.e., segmentectomy and wedge resection) are chosen in patients with limited pulmonary reserve, or recently, in those with small peripheral tumors without nodal disease for curative intent. The indications for curative-intent sublobar resection are discussed later. On occasion, sleeve lobectomy is required when the tumor protrudes into the main-stem bronchus. A concern with sleeve lobectomy is that it might be an inferior oncologic resection compared with pneumonectomy; however, sleeve lobectomy has proved to be superior to pneumonectomy with regard to long-term outcome, with lower morbidity and mortality.15,16
Mobilization and Hilar Dissection
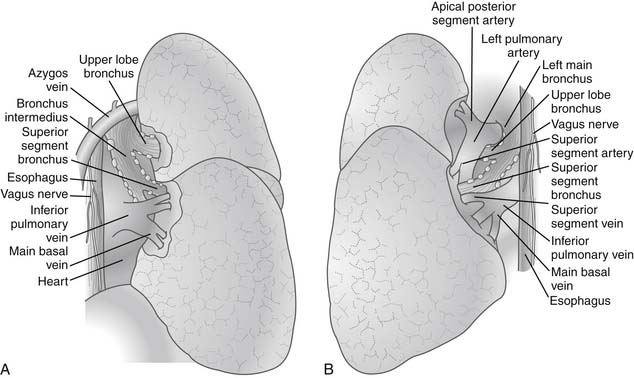
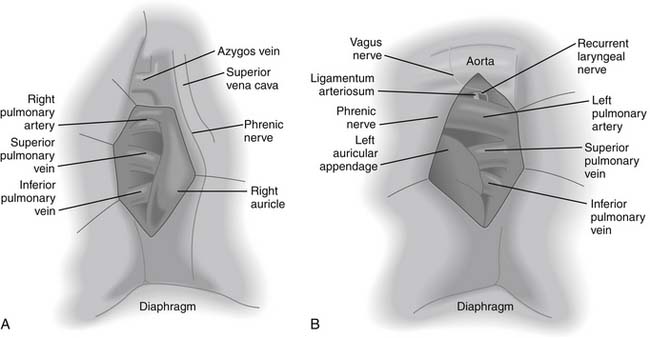
After the pleural cavity is entered, careful inspection of the pleural space is performed. Any pleural fluid is sampled for cytology and culture. All lobes of the lung are then palpated to assess the extent of the disease, to identify unexpected pleural or parenchymal lesions, and to confirm resectability. The inferior pulmonary ligament is incised up to the inferior pulmonary vein, and the mediastinal pleura of the hilum is incised circumferentially to totally mobilize the lung. Care must be taken to prevent injury to the phrenic nerve by electrocautery when the anterior hilum is opened. The posterior views of right and left pulmonary hila are shown in Figure 17-1.
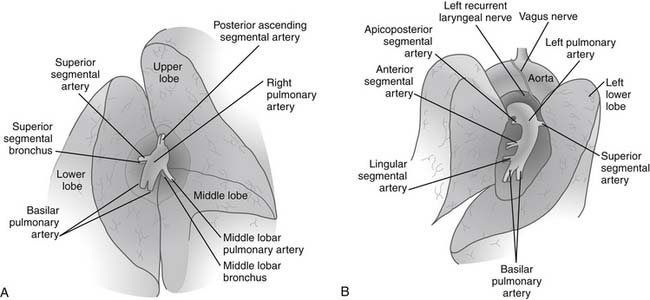
The hilar vascular structures (the main pulmonary artery and the superior and inferior pulmonary veins) are identified and dissected. The fissures between the lobes are often not completely open, so dissection is required to reach the interlobar structures. The incomplete fissures usually necessitate a combination of sharp and blunt dissection. Care must be taken to avoid violation of the tumor if an extension of the tumor from one lobe to another is seen. The interlobar views of right and left lungs are shown in Figure 17-2.
Pneumonectomy
As anatomic lobectomy has become the standard form of curative resection for bronchogenic carcinoma, pneumonectomy has become less prevalent. Pneumonectomy is reserved for central lesions, traditionally, but even in patients with tumor invasion of the main-stem bronchus or bronchus intermedius, sleeve lobectomy is the procedure of choice, with acceptable morbidity, mortality, and long-term survival.17 After careful assessment and patient selection, pneumonectomy is used for patients with centrally located NSCLC that cannot be completely resected using bronchoplastic procedures.
Hilar Dissection
When the tumor is in close proximity to the proximal pulmonary artery, and thus it is not possible to obtain a sufficient margin to apply a stapler, the pericardium should be opened to obtain additional length for division (Fig. 17-3). On the right side, mobilization of the superior vena cava is often required to encircle the proximal right main pulmonary artery. On the left side, division of the ligamentum arteriosum produces additional length for division of the left main pulmonary artery proximally. During this maneuver, special attention should be paid to avoiding injury to the left recurrent laryngeal nerve, if the nerve is not involved by the tumor. Dissecting proximally after opening the pericardium at its reflection, one can divide the left pulmonary artery at its origin. It is important not to impinge on the right pulmonary artery when applying traction to staple the left main pulmonary artery.
Management of Bronchus
The division of the main-stem bronchus should be performed as proximally as possible (3 to 5 mm from the carina) to avoid creating an unnecessarily long bronchial stump. On the right side, the carina and the anticipated point of bronchial division is often visualized easily. The division of the left main-stem bronchus requires some traction on the bronchus, because the carina is poorly visualized from the left side. Peribronchial tissues and lymph nodes around the bronchus are cleared to better expose the bronchus, but an excessive devascularization should be avoided to preclude poor healing that could lead to postoperative BPF. In patients who received induction radiotherapy, the stump of the right main bronchus should be covered by a pericardial fat pad, mediastinal pleura, pericardium, intercostal muscle flap, or even omentum. Although it is generally desirable to protect the right main bronchial stump, it is not always necessary on the left side because the left main stump retracts into mediastinal tissues after division. Darling and colleagues18 reported the rates of BPF around 13% on the right side and 5% on the left side, with 8% overall.
Sublobar Resection (Segmentectomy and Wedge Resection)
Sublobar resection was originally developed for the surgical management of infectious lung diseases such as tuberculosis and bronchiectasis, in which bilateral or multisegmental disease is frequently seen. For the treatment of NSCLC, this has generally been the option for patients with poor pulmonary function who may not tolerate anatomic lobectomy, because limited resections have demonstrated a higher local recurrence rate and worse long-term outcome than anatomic lobectomy.19 However, recent evidence suggests that sublobar resection, especially anatomic segmentectomy, may in fact be the surgical procedure of choice, not only for patients with poor cardiopulmonary reserve but also for those with early stage NSCLC at low risk.20 In general, anatomic segmentectomy is preferred over simple wedge resection to clear the lymphatics draining the tumor bed. Generous wedge resection with cancer-free margins may be acceptable for small, peripheral, “nonsolid” bronchioloalveolar carcinoma.21 Sampling or formal dissection of hilar and mediastinal lymph nodes with intraoperative frozen-section pathology should be performed to confirm any nodal disease. If proven to be positive for nodal disease intraoperatively, the surgical resection should be converted to anatomic lobectomy whenever possible.
Surgical Principles
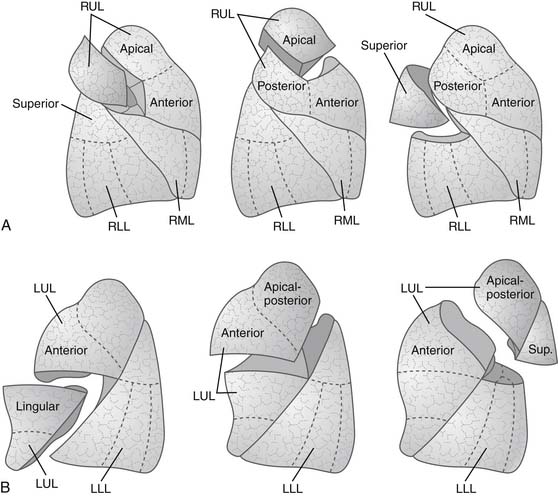
The landmark of a diseased segment used to guide resection is its bronchus. Thus identification of the bronchus of the diseased segment is a key step. Therefore, the division of segmental structures tends to begin with the segmental arterial branch, because this often allows good exposure of the segmental bronchus, which runs along with the segmental artery. The segmental vein is best divided last, after identification of the intersegmental plane. Intersegmental veins, the venous drainage of adjacent segments, are usually preserved. The separation at the intersegmental lung parenchymal plane is performed with differential lung deflation and inflation. First, the segmental bronchus of the diseased segment is clamped in a deflated state. Then the lung is inflated, and the demarcation line can be identified because the diseased segment remains airless while other parts of the lung are expanded. Alternatively, the lung is expanded first, followed by clamping the bronchus of the diseased segment, then the lung is deflated to demarcate the diseased segment that remains expanded. Manual monofilament absorbable interrupted suturing (4-0 polydioxanone [PDS] or Maxon) is usually used for the bronchial closure. Stapling may be performed if sufficient length of the diseased segmental bronchus is available. In this case, care must be taken to avoid compromising adjacent segmental bronchi. Blunt or sharp dissection of the intersegmental plane is traditionally performed by scissors, a sponge on a stick, or the surgeon’s fingers. Small bridging veins or bronchi are individually clipped or ligated for division. Stapling the intersegmental plane is also applicable and desirable, and this is achievable if the resection is extended into an adjacent segment to achieve an adequate margin from the tumor. Any air leaks from the dissecting plane are controlled by nonabsorbable suturing. Various types of segmentectomy are shown in Figure 17-4.
Mediastinal Lymph Node Dissection
Rationale
Recent advances in mediastinal staging with computed tomography (CT), positron emission tomography (PET), and endoscopic ultrasound bronchoscopy with real-time guided fine-needle aspiration (EBUS-FNA) have improved diagnostic accuracy.22,23 However, there is no doubt that intraoperative mediastinal lymph node staging remains the gold standard, and it is an integral part of the surgical treatment of lung cancer to determine accurate nodal (N) status, which is a significant outcome predictor.24 Generally, lymph node sampling indicates the removal of macroscopically abnormal lymph nodes. When biopsies are performed from all lymph node stations, it is said to be systematic sampling. If systematic removal of all the mediastinal tissue containing lymph nodes within anatomic landmarks is performed, it is referred to as complete lymph node dissection. Bilateral mediastinal and cervical lymph node clearance is referred to as extended lymph node dissection.25 It has been a subject of controversy as to whether complete lymph node dissection is superior to systematic sampling in diagnostic accuracy and long-term survival. These issues are discussed later. The classification and graphic representation of lymph node levels for lung cancer staging by the American Joint Committee on Cancer (AJCC) and the Union Internationale Contre le Cancer (UICC)26 are shown in Figure 17-5.
SURGICAL TREATMENT OF NON–SMALL CELL LUNG CANCER
The International Association for the Study of Lung Cancer (IASLC) staging committee recently published proposals for the revision of the TNM classification scheduled for 2009.27 The proposed definitions of TNM descriptors are shown in Table 17-1. Specific surgical issues related to each stage (T, N, and M) are discussed here.
Table 17–1 Proposed Definitions for T, N, and M Descriptors by the International Association for the Study of Lung Cancer (IASLC) www.lww.com
| T (Primary Tumor) | |
| TX | Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤ 3 cm in greatest dimension, surrounded by lung or pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus)∗ |
| T1a | Tumor ≤ 2 cm in greatest dimension |
| T1b | Tumor > 2 cm but ≤ 3 cm in greatest dimension |
| T2 | Tumor > 3 cm but ≤ 7 cm or tumor with any of the following features (T2 tumors with these features are classified T2a if ≤ 5 cm) |
| T2a | Involves main bronchus, ≥2 cm distal to the carina |
| T2b | Invades visceral pleura |
| Associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung | |
| Tumor > 3 cm but ≤ 5 cm in greatest dimension | |
| Tumor > 5 cm but ≤ 7 cm in greatest dimension | |
| T3 | Tumor > 7 cm or one that directly invades any of the following: chest wall (including superior sulcus tumors), diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium; or tumor in the main bronchus < 2 cm distal to the carina∗ but without involvement of the carina; or associated atelectasis or obstructive pneumonitis of the entire lung or separate tumor nodule(s) in the same lobe |
| T4 | Tumor of any size that invades any of the following: mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina; separate tumor nodule(s) in a different ipsilateral lobe |
| N (Regional Lymph Nodes) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension |
| N2 | Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s) |
| N3 | Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) |
| M (Distant Metastasis) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Separate tumor nodule(s) in a contralateral lobe; tumor with pleural nodules or malignant pleural (or pericardial) effusion† |
| M1b | Distant metastasis |
∗ The uncommon superficial spreading tumor of any size with its invasive component limited to the bronchial wall, which may extend proximally to the main bronchus, is also classified as T1.
† Most pleural (and pericardial) effusions with lung cancer are due to tumor. In a few patients, however, multiple cytopathologic examinations of pleural (pericardial) fluid are negative for tumor, and the fluid is nonbloody and is not an exudate. Where these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging element and the patient should be classified as T1, T2, T3, or T4.
(Adapted from Goldstraw P, Crowley J, Chansky K, et al. J Thorac Oncol 2000;2:706–14.)
T Category: T1 and T2
T1 and T2 tumors will be subcategorized in the next edition of the TNM staging according to the proposals by the IASLC Lung Cancer Staging Project.28 The proposed changes in the T classification in the T1 and T2 categories are as follows: (1) T1 will be T1a if the tumor is 2 cm or less, and T1b if the tumor is greater than 2 cm but not greater than 3 cm; (2) T2 tumors are greater than 3 cm, and they will be T2a if equal to or less than 5 cm, and T2b if greater than 5 cm and equal to or less than 7 cm. Tumors greater than 7 cm will be moved into the T3 category (see Table 17-1).
For the T1 and T2 categories, the surgical procedure of choice is essentially the same if patients are medically fit and have good cardiopulmonary reserve; anatomic lobectomy is the preferred mode of resection. However, recent refinements of prognostication suggest that sublobar resection (segmentectomy or wide wedge resection) can be performed on patients with small, peripheral, early-stage NSCLC without jeopardizing the clinical outcome. Additional multicenter trials comparing lobectomy with lesser resections are under way. On another front, a growing body of evidence suggests that surgical resection alone is not adequate for the treatment of large-sized NSCLC without any nodal disease.2
Sublobar Resection versus Lobectomy for Node-Negative, Small-Size NSCLC
Historically, Jensik and coworkers13 reported their 15-year experience of segmental resections of peripheral lung cancer as a curative-intent procedure in 1973. The 5-year survival rate of 69 patients who underwent curative resections by segmentectomy was 56%. Of these, six (9%) developed local recurrence in the primary lobe. The authors concluded that segmentectomy can be a valid procedure for selected, peripherally located lung cancer. In the early 1980s, the Lung Cancer Study Group (LCSG) performed a multicenter randomized clinical trial of lobectomy versus a lesser resection by wedge or segmentectomy in T1N0 NSCLC in patients presenting with small peripheral tumors, and the report suggested a threefold increase in the incidence of local recurrence in patients treated by resections smaller than lobectomy.19 Therefore, the authors recommended that the use of sublobar resection should be limited to poor-risk patients with compromised pulmonary function.
However, more recent evidence suggests that radical, curative-intent sublobar resection can be offered for clinical T1N0 NSCLC even in good-risk patients. The survival outcome and locoregional recurrence rates of anatomic segmentectomy in comparison with lobectomy are summarized in Table 17-2.19,29–32 Although most of the evidence is from retrospective studies comparing the outcome of segmentectomy with that of lobectomy, several key factors have been identified to obtain optimal results by anatomic segmentectomy. The factors include (1) surgical margins, (2) tumor size, and (3) lymph node assessment.
Table 17–2 Long-Term Outcome and Local Recurrence Rate of Sublobar Resection in Comparison to Lobectomy in Patients with Early-Stage Non–Small Cell Lung Cancer
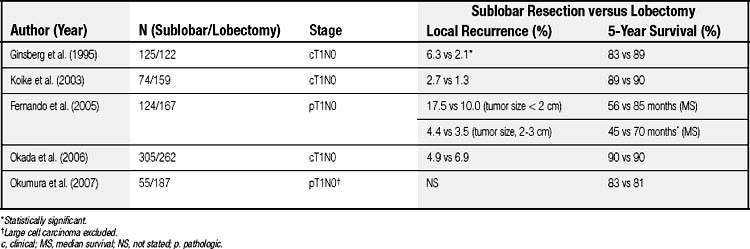
It is clear that positive surgical margins must be avoided to achieve complete resection; however, it remains unresolved as to what is the optimal adequate surgical margin (i.e., the distance from the tumor to the cut margin) for localized tumors in the setting of segmentectomy. Sawabata and colleagues33 conducted a prospective study to define optimal surgical margins in excision of NSCLC. In the study, the surgical margins were microscopically 100% negative for malignancy when the margin distance was greater than 20 mm, or when the resected tumors had a margin distance greater than the maximum tumor diameter (a margin-to-tumor size ratio > 1). Schuchert and coworkers34 reported that the local recurrence rate was significantly higher in patients with a margin-to-tumor ratio less than 1, than in those with the ratio greater than 1 (25% versus 6.2%). In the study, the mean surgical margin in patients with local recurrence was 12.8 mm, and that in those without local recurrence was 18.6 mm. Therefore, lobectomy or extended segmentectomy should be performed when an adequate surgical margin (preferably 2 cm) cannot be obtained, especially if tumors are located centrally. Intraoperative pathologic assessment of surgical margins should generally be performed to confirm the margins.
Tumor size is also an important factor when segmental resection is considered. A large tumor size is associated with increased risk of local recurrence and distant metastasis. Fernando and colleagues reported that there was no difference in survival between sublobar resection and lobar resection groups when the tumor size was smaller than 2 cm in pathologic T1N0 NSCLC, whereas the median survival was significantly better in the lobar resection group when tumor size was greater than 2 cm (68.7 versus 50.6 months).30 Okada and colleagues35 reported the 5-year cancer-specific survivals of patients with pathologic stage I disease with tumors of 2 cm or less and 2.1 to 3 cm in diameter were similar: 92.4% and 87.4% after lobectomy, and 96.7% and 84.6% after segmentectomy, respectively. On the other hand, in patients with a tumor greater than 3 cm in diameter, survivals were significantly worse in the segmentectomy group than in the lobectomy group (62.9% versus 81.3%). Bando and colleagues36 reported a higher locoregional recurrence rate of segmentectomy in patients with tumors 2.1 to 3 cm than in those with tumors 2 cm or smaller. In the report by Schuchert and associates,34 recurrence was observed more frequently in stage IB patients than stage IA patients. Jones and coworkers37 reported no locoregional recurrence in 43 patients with pathologic T1N0 NSCLC who underwent segmentectomy. El-Sherif and colleagues38 recently reviewed their experience of sublobar resection versus lobectomy and demonstrated that the disease-free survival rate of sublobar resection was equivalent to that of lobectomy in patients with stage IA NSCLC, but in those with stage IB NSCLC, survival was worse in the sublobar resection group. Several recent series have also reported comparable long-term outcomes of segmentectomy and lobectomy in small (tumor size, <2 cm) T1N0 NSCLC.29,31,39 Based on the current evidence, patients with early-stage small NSCLC (preferably 2 cm or less in size) may undergo anatomic segmentectomy if negative nodal disease is confirmed.
Intraoperative lymph node assessment is an integral part of sublobar resection. Miller and colleagues40 evaluated the frequency of lymph node metastasis in patients who underwent resection of NSCLC 1 cm or less. The tumors were located in the periphery in 89 patients, centrally in three, and endobronchially in eight. Of these, seven (7%) had lymph node metastasis (N1 = 5 and N2 = 2). In the prospective nonrandomized trial of sublobar resection reported by Okada and colleagues,31 of 305 patients with clinical T1 (tumor size, <2 cm) N0 NSCLC who were assigned to undergo sublobar resection, 20 (7%) were found to have lymph node metastasis (N1 = 11 and N2 = 9) intraoperatively, so the procedure was converted to lobectomy. Therefore, even for tumors smaller than 1 cm, intraoperative hilar and mediastinal lymph node sampling or dissection is recommended whenever sublobar resection is performed.
Wedge resection has appeared to be suboptimal for the surgical treatment of lung cancer except for localized, small-size pure bronchioloalveolar carcinoma because of a higher local recurrence rate. Landreneau and associates41 documented high local recurrence rates in patients with T1N0 NSCLC who underwent wedge resection (wedge versus lobectomy: 24% versus 9%). Sienel and coworkers42 analyzed local recurrence rates in patients with pathologic T1N0 NSCLC who underwent either segmentectomy or wedge resection. Segmentectomies were followed by systematic lymph node dissection, and wedge resections by lymph node sampling. The local recurrence rate was significantly higher in the wedge resection group than in the segmentectomy group (55% versus 16%), and the difference of recurrence rates was significant even in patients with tumors 2 cm or less (40% versus 11%). The observed higher locoregional recurrence rates may be partly explained by an inadequate lymph node assessment, because sampling of interlobar lymph nodes or segmental hilar nodes is often impractical or impossible, resulting in the missing of positive nodes and false downstaging. Wedge resection may also leave draining lymphatic vessels containing cancer cells behind in the residual diseased segment, thus increasing the risk of local recurrence. Therefore, anatomic segmentectomy, but not wedge resection, is recommended for patients with early-stage small NSCLC when sublobar resection is considered. If anatomic segmentectomy might not be tolerated because of poor cardiopulmonary reserve, wedge resection combined with radiotherapy or brachytherapy could be an alternative approach to reduce the risk of local recurrence.30,43
Although the topic of adjuvant therapy is not in the scope of this chapter, recent evidence suggests that patients with early-stage, resectable NSCLC (including N1 disease) appear to benefit from adjuvant or neoadjuvant therapy.2,44–47 This topic is discussed in Chapter 19.
T Category: T3
The T3 category includes (1) tumors greater than 7 cm; (2) additional nodules in the primary lobe; (3) tumors of any size invading the chest wall, diaphragm, mediastinal pleura, or parietal pericardium; (4) tumors in the main-stem bronchus less than 2 cm from the carina without invading the carina; and (5) tumor-associated atelectasis or obstructive pneumonitis of the entire lung (see Table 17-1). Some of these subsets are discussed with respect to their specific surgical considerations later.
Tumors Invading the Chest Wall
Technical details and the outcome of this subset are reviewed in Chapter 20. NSCLC invading the chest wall is usually peripheral in location, so mediastinal lymph nodes are less likely to be involved. The degree of chest wall involvement with tumor varies from the parietal pleura to the muscles and ribs of the chest wall. Those with NSCLC involving the chest wall, but without mediastinal node disease, are good candidates for surgical treatment whenever medically fit. Postoperative mortality has continued to be reduced, in recent reports ranging between 0% to 6.3%.48–54 In general, factors affecting long-term survival include (1) completeness of resection,48,50,54 (2) extent of invasion of the chest wall,49,50,54 and (3) nodal status.48,50,51,53,54 In patients with incomplete resection (macroscopic or microscopic disease) or unresectable tumors, the 5-year survival was essentially zero.48,54 It is a matter of controversy as to whether en bloc (full-thickness chest wall) resection is necessary for all depths of tumors invading the chest wall, but some authors advocate a full-thickness resection to achieve complete tumor clearance.50,51,53 The roles of chemotherapy and radiotherapy for this group of patients are still uncertain and clearly require further investigation.53
Superior Sulcus Tumors
Advances in surgical techniques and combined-modality approaches recently led to the conduct of two prospective multicenter phase II clinical trials, where induction chemoradiotherapy was performed followed by surgical resection in patients with superior sulcus NSCLC.55,56 The North American Intergroup trial (Southwest Oncology Group 9416, INT 0160) report by Rusch and colleagues55 suggested that both T3N0-1 and T4N0-1 tumors benefit from preoperative chemoradiation with high response rates by pathologic examination (61% with complete pathologic response or minimal microscopic tumor) and high complete resection rates (76% overall, and 94% of patients who underwent thoracotomy). The median survival was 33 months for all patients, and 94 months for the patients who achieved an R0 resection. Kunitoh and coworkers56 reported mature results of induction chemoradiotherapy followed by surgical resection in patients with T3 (n = 56) or T4 (n = 20) NSCLC (Japan Clinical Oncology Group Trial, JCOG 9806). Of these, 57 patients (76%) underwent surgical resection and, again, a high R0 resection rate (68% overall, and 90% of patients who received surgical resection) was achieved. The overall 5-year survival rate was 56%. In both studies, the pattern of cancer recurrence was predominantly distant, and the brain is the leading organ of relapse. In the INT 0160 trial, 19 of 57 patients (41%) who developed recurrence had brain metastasis only, and 5 of 39 patients (13%) did so in the JCOG 9806 trial.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree