Chapter 118
Lower Extremity Amputation
Techniques and Results
John F. Eidt, Venkat R. Kalapatapu
Amputation of a damaged limb is one of the oldest and, arguably, most effective surgical procedures. A properly performed amputation holds the promise of pain relief for patients with advanced ischemia, control of infection in the setting of extremity sepsis, and cure of malignancy confined to the limb. Unfortunately, vascular surgeons often regard amputation as the terminal battle in the war against atherosclerosis and an admission of personal failure. As such, amputation is too frequently relegated to the least experienced member of the surgical team to perform at the end of the day with insufficient guidance. This chapter emphasizes the therapeutic value of a well-done amputation and describes the technical details essential to successful amputation surgery.
As one of the oldest surgical procedures, amputation has been refined over many centuries.1 Progress in amputation surgery was hindered by early surgeons’ inability to manage the critical triad of infection, hemorrhage, and pain. Before antisepsis was introduced by British surgeon Joseph Lister in 1867, more than half of all amputees died from infection. Before the introduction of general anesthesia, surgical speed was the only way to limit pain and hemorrhage. A variety of novel amputation devices were developed by 16th-century French and German barber-surgeons to achieve rapid removal of the offending limb. The introduction of vessel ligatures by Ambrose Paré in 1529 put an end to the barbaric use of mass cauterization with scalding oil. Another technical landmark was the introduction of the hemostatic tourniquet by another French surgeon, Morell, who is said to have first used the technique successfully in the battle of Besançon in 1674.1
General Principles
Regardless of amputation level, a number of general principles apply (Box 118-1). The surgeon must be satisfied that there is sufficient arterial perfusion at the amputation level to sustain healing. In addition, it is important to ensure the structural integrity of the bony architecture of the residual limb. For example, in some patients with diabetes and Charcot’s degenerative osteoarthropathy of the foot, formal transtibial amputation may be superior to complex foot salvage procedures. Meticulous attention to detail and gentle handling of soft tissues are vital in creating a well-healed and functional amputation stump. The use of skin hooks or fine-toothed forceps to retract the skin is preferable to the use of crushing instruments such as DeBakey-type forceps. Some surgeons condemn the use of any grasping instruments and rely on gentle finger traction alone. Hemostasis is best obtained with fine suture ligatures, and the use of electrocautery should be minimized. The knife blade should be maintained perpendicular to the skin surface to avoid a bevel, and the skin should not be separated from the underlying fascia to prevent ischemia. In planning the amputation, the surgeon should avoid placing surgical scars on weight-bearing surfaces. If possible, the skin should be separated from the cut surface of bone by a myofascial layer to prevent the formation of adherent scar, which may be a source of pain. Skin flaps should be approximated without tension. Although the maintenance of limb length is generally desirable, removal of all nonviable and infected tissue is a higher priority. Excessively redundant soft tissue interferes with proper prosthetic fit and should be avoided. However, an amputation site has a remarkable capacity for remodeling, so compulsive trimming of small dog-ears and other minor cosmetic irregularities is usually best avoided. The use of a hemostatic tourniquet is beneficial in reducing blood loss.2 Antagonistic muscle groups should be stabilized to prevent muscle atrophy and skeletal misalignment.3 The term myoplasty is used to describe the suture fixation of antagonistic muscle groups directly to each other, as opposed to myodesis, in which the musculotendinous unit is sutured directly to bone. Sharp bone edges should be contoured, and bony prominences should be beveled. Nerves should be sharply transected proximal to weight-bearing surfaces to avoid painful neuroma formation. Excessive periosteal stripping is generally contraindicated because it can result in the formation of ring sequestra or bony overgrowth. Drains may be used and are usually removed after 1 to 2 days.
Postoperatively, surgeons should be mindful that accidental injury to the recent amputation stump is a frequent cause of failure; this may be preventable by the liberal use of both rigid and soft protective dressings. In most cases, weight bearing should be completely avoided until the wounds are well healed. All amputation patients should receive appropriate prophylaxis against deep venous thrombosis (DVT). We prefer low-molecular-weight heparin administered subcutaneously until ambulation is initiated.4 All patients receive perioperative intravenous prophylactic antibiotics appropriate for skin flora.5
When approaching an infected limb, several unique factors must be considered. Mechanical barriers, such as impervious plastic sleeves or iodinated adhesive skin drapes, should be used to isolate infected areas. Open “drainage” or guillotine amputation should be considered in most cases of gross infection.6–10 Delayed primary closure after the septic process is controlled (two-stage approach) may preserve limb length. Antibiotic coverage should be based on Gram stain and culture results.
Amputation Types
Toe and Ray Amputation
Anatomy
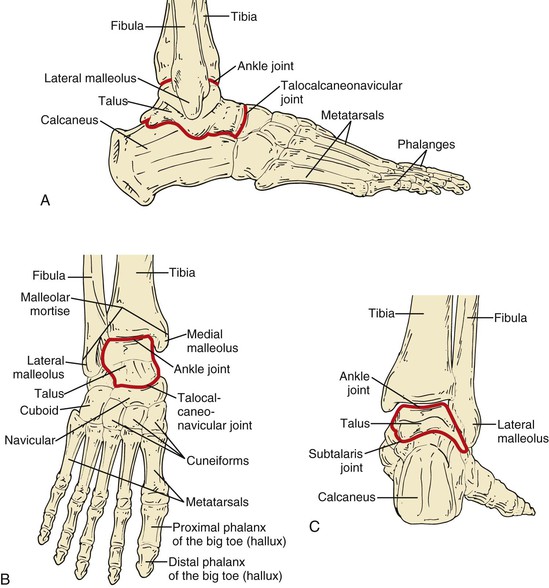
There are two phalanges in the first toe and three in each of the remaining four toes (Fig. 118-1). The interphalangeal and metatarsophalangeal joints are hinge joints. Each has an articular capsule and medial and lateral collateral ligaments. The plantar surface of the articular capsule is strengthened to form a fibrous plate, the plantar ligament, which limits toe extension. The flexor hallucis longus and flexor digitorum longus tendons insert on the distal phalanges. The flexor hallucis brevis inserts on the proximal phalanx of the first toe. There are typically two sesamoid bones contained within the flexor hallucis brevis tendon that articulate with the plantar surface of the first metatarsal head.
Technique: Toe Amputation
Toe amputation is appropriate for wounds limited to the middle and distal toe and not involving the skin over the metatarsal head. More proximal wounds typically necessitate ray amputation. Ankle or digital nerve block is preferred for most toe amputations, but spinal, epidural, or general anesthesia may be chosen. Care should be taken to avoid direct puncture of the pedal arteries or excessive infiltration of anesthetic in the dysvascular foot because of the risk of exacerbating ischemia. Some surgeons avoid the use of ankle and digital blocks in the setting of local foot sepsis for fear of spreading cellulitis. The foot and toes should be scrubbed vigorously with an antiseptic soap. Iodine-impregnated adhesive drapes are impractical on the toes, but a surgical glove may be useful to isolate a necrotic or septic area.
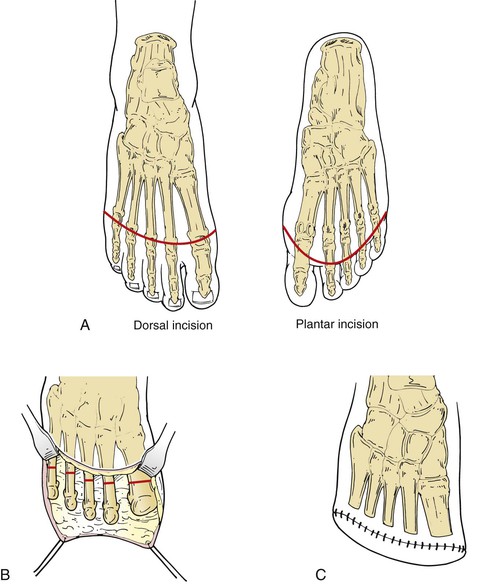
Partial or complete amputation of the toe can be performed. Almost any incision that results in tension-free coverage of the transected bone can be chosen; however, the simplest technique for complete amputation of the toe is based on the racket incision, so named because its shape is similar to that of a tennis racket (Fig. 118-2). For amputation of the first and fifth toes, we prefer to orient the handle of the racket on the medial or lateral surface of the respective metatarsal head. For the second, third, and fourth toes, the handle of the racket is oriented longitudinally along the dorsal surface of the digit. For partial amputation of the toe, a transverse or vertical fish-mouth skin incision may be used.
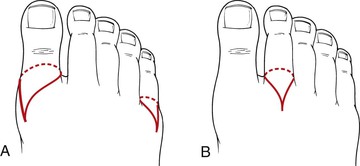
Figure 118-2 A, Racket incision for first and fifth toe amputation. B, Racket incision for second, third, or fourth toe amputation.
The flexor and extensor tendons are divided at the level of the skin incision and allowed to retract. The bone is divided at the appropriate level. If disarticulation is desired, a No. 15 knife blade is inserted into the joint, and the joint capsule is incised sharply to avoid injury to surrounding soft tissue. Because the articular cartilage is poorly vascularized, we prefer to excise the cartilage with a rongeur, although there are no data to support this maneuver. We also gently shape the exposed bone to eliminate any bony projections that might lead to wound or skin problems. The wound is irrigated vigorously, and careful hemostasis is obtained. Excessive use of electrocautery is avoided. The skin is closed without tension in one layer using interrupted, nonabsorbable monofilament suture. Weight bearing is completely prohibited until adequate healing is observed.
Technique: Ray Amputation
Ray amputation involves amputation of the toe along with all or part of the corresponding metatarsal head. Although a single isolated ray amputation sometimes proves durable, multiple ray amputations narrow the foot excessively and create biomechanical instability. This increases the amount of weight that must be borne by the remaining metatarsal heads and can lead to new areas of increased pressure, callus formation, and ulceration. Ray amputation may be useful in the treatment of blue toe syndrome caused by microatheroembolism involving the entire toe if there is insufficient skin to cover the exposed metatarsal head. Ray amputation may also be useful in the treatment of mal perforans ulcers in the neuropathic diabetic foot, especially in the setting of osteomyelitis of the metatarsal head. Because a hallux valgus deformity commonly develops following isolated second toe amputation, complete second ray amputation may be preferable because it reduces the angle between the first and third metatarsals.
In a typical ray amputation, we modify the racket incision by extending the handle of the racket to provide sufficient exposure of the shaft of the metatarsal (Fig. 118-3). The extensor tendons are divided and allowed to retract, and the shaft of the metatarsal is transected with a power saw. The distal segment of metatarsal is engaged with a bone hook, and distal traction is applied while the remaining plantar soft tissue attachments are divided. Care is taken to stay close to the bone to avoid injury to adjacent digital arteries and nerves. Sesamoid bones are excised, and the flexor tendons are transected under tension to facilitate retraction.
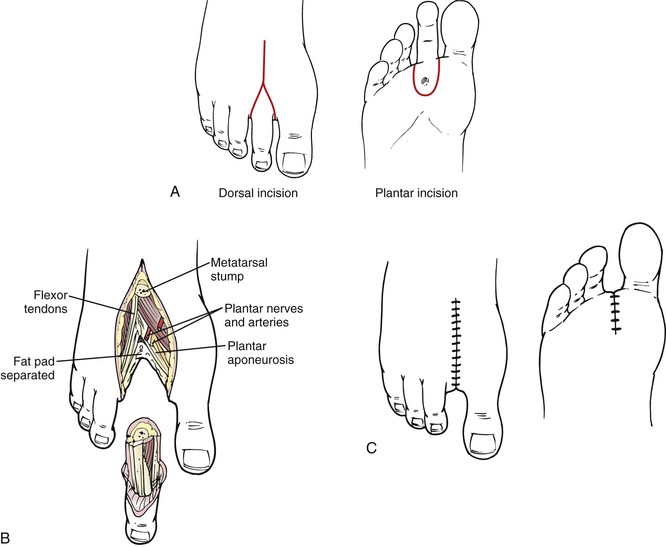
Figure 118-3 A, Ray amputation incision. Note the plantar extension to include a mal perforans ulcer. B, Transection of the metatarsal shaft. C, Closure with nonabsorbable suture under no tension.
In comparison to the lateral four rays, the first metatarsal and great toe contribute disproportionately to the mechanics of normal ambulation. In a normal stride, the foot contacts the ground on the posterolateral aspect of the heel. The weight is transferred progressively along the lateral plantar surface of the foot and then across the metatarsal heads from lateral to medial. Finally, the foot lifts off primarily from the first metatarsal head and the great toe. Although near-normal foot mechanics can be achieved after amputation of one of the lateral four rays, amputation of the great toe and first metatarsal head markedly alters normal ambulation and may result in destructive forces within the architecture of the foot. Recurrent ulceration following first ray amputation has been reported in up to 60% of patients.11 Some orthopedic surgeons recommend formal transmetatarsal amputation of all five rays rather than isolated first ray amputation.12 The contrarian viewpoint holds that transmetatarsal amputation may require revision or reamputation in 20% to 40% of patients and that above-ankle amputation is necessary in one of five patients.13,14 Thus, there is a lack of consensus in the literature, and the final decision between first ray amputation and complete transmetatarsal amputation should be individualized, based on patient and surgeon preferences.
Postoperative Considerations
Weight bearing is prohibited until the wound is thoroughly healed. We usually use a simple bulky soft dressing to protect the wound. A rocker-bottom shoe with a metatarsal bar that unloads pressure from the toes may be effective in selected patients. Approximately 25% of toe amputations fail to heal and require additional amputation at a higher level.15 Of these higher amputations, approximately 40% are at the transtibial level.16 With the use of appropriate orthoses and foot care, many patients achieve durable ambulation following toe or ray amputation.
Transmetatarsal Amputation
Anatomy
The metatarsal heads are stabilized by a series of segmental deep transverse metatarsal ligaments that connect the plantar ligaments of the adjacent metatarsophalangeal joints (see Fig. 118-1). Proximally, the metatarsals articulate with the three cuneiforms—medial, intermediate, and lateral—and the cuboid bone. These bones are connected by the dorsal and plantar tarsometatarsal and the interosseous cuneometatarsal ligaments. The tarsometatarsal, or Lisfranc’s, joint connects the midfoot to the forefoot.
Technique
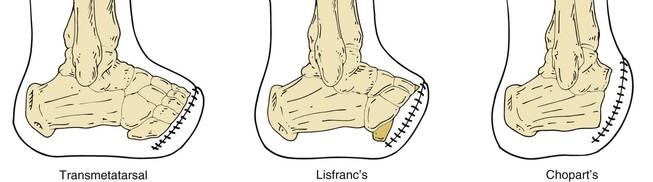
Transmetatarsal amputation (TMA) is appropriate for wounds involving the entire forefoot. TMA may be preferable to isolated first ray amputation in selected patients because TMA has better healing rates and, owing to improved foot mechanics, better rehabilitation rates. TMA also offers the benefits of full limb length, normal shoe wear, and near-normal ambulation. A one-stage TMA should generally not be undertaken in the setting of severe forefoot infection. In this situation, a guillotine débriding amputation should be considered, followed by formal wound closure after control of sepsis. A partial-thickness skin graft may be an effective method to salvage an open TMA wound if it is restricted to non–weight-bearing surfaces. TMA is contraindicated if there is excessive bone deformity in the midfoot and hindfoot, which would not be structurally sound.
The entire lower leg and foot are prepared and draped. A pneumatic tourniquet is placed on the lower leg. A latex bandage is used to exsanguinate the foot before tourniquet inflation, unless there is significant forefoot sepsis. The incision extends from a point just medial to the first metatarsal head transversely across the dorsum of the foot at the level of the distal metatarsal shaft and ends on the lateral side of the fifth metatarsal head (Fig. 118-4).17 The incision then extends at right angles onto the plantar surface of the foot, creating a plantar flap that extends up to the base of the toes. The extensor tendons and sheaths are divided at, or slightly proximal to, the level of the dorsal skin incision to expose the metatarsal shafts. The periosteum is divided just proximal to the metatarsal heads, and the metatarsals are transected using a sharp power saw. We prefer to divide the metatarsals in a gentle curve from medial to lateral, with each successive shaft being 3 to 5 mm shorter than the previous one. Some surgeons advocate transecting the metatarsal shaft with a 30- to 45-degree plantar-oriented bevel to facilitate lift-off during ambulation. Following transection of the bone, the distal metatarsal fragments can be grasped with a bone clamp or bone hook and retracted distally while the plantar myofascial attachments to the metatarsal heads are sequentially divided. The flexor tendons and sheaths are poorly vascularized and should be excised individually, along with other fibrocartilaginous structures on the plantar flap, taking care not to incise too deeply. The tourniquet is deflated, and bleeding is controlled with fine suture ligatures. Use of electrocautery is minimized. The wound is irrigated, and the plantar flap may be trimmed to fit if necessary. The wound is closed in two layers with interrupted absorbable sutures in the fascia. The skin is closed loosely with interrupted nonabsorbable monofilament sutures or staples. In order to minimize the risk of equinus deformity, some surgeons recommend dividing or lengthening the Achilles tendon at the time of transmetararsal amputation. Randomized data supporting this practice are not available but anecdotal evidence suggests that the risk of future forefoot ulceration is reduced with this technique.
Postoperative Considerations
A posterior plaster splint that maintains the foot at 90 degrees is applied. Weight bearing is prohibited until adequate healing is observed, usually after 3 to 4 weeks. TMA is a relatively durable procedure following initial healing. Primary healing can be expected in 50% to 75% of patients following TMA.18–21 Revision to a higher level is necessary in approximately 25% to 40% of TMAs.15 A vacuum dressing may be effective in avoiding more proximal amputation.22 With appropriate orthoses, patients can be expected to ambulate with an almost imperceptible gait alteration following complete healing.
Midfoot and Hindfoot Amputations
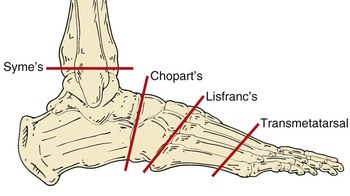
Three proximal foot amputations may be appropriate in vascular surgery patients (Fig. 118-5): Lisfranc’s tarsometatarsal disarticulation, Chopart’s midfoot amputation, and Syme’s amputation.23,24 Two other hindfoot amputations that are rarely employed are Boyd’s and Pirogoff’s amputations.17,23,25,26 These hindfoot amputations are performed primarily in children to preserve length and growth centers.
The use of midfoot and hindfoot amputations in diabetic patients remains controversial. Many surgeons recommend formal transtibial (below-knee) amputation if TMA cannot be performed or has failed. Others suggest that increased limb salvage rates can be achieved with the use of unconventional foot amputations.25,27 Recently, it has been shown that the preservation of leg length may give diabetic patients a survival advantage. Stone and coworkers reported that diabetic patients undergoing proximal foot amputations had better function and survival than those undergoing below-knee amputations (BKAs).26 These findings were not observed in dialysis-dependent diabetic patients, however.
Anatomy
The tibia and the fibula articulate with the talus via the talocrural joint. The ligaments that join these bones are the deltoid, anterior and posterior talofibular, and calcaneofibular ligaments. The talus articulates with the calcaneus at the subtalar joint. The medial and lateral talocalcaneal ligaments and the cervical ligament support this joint. The talocalcaneonavicular joint is a multiaxial joint supported by the talonavicular and plantar calcaneonavicular ligaments. Supination (inversion) at this joint is produced by the tibialis anterior and posterior. Pronation (eversion) is produced by the peroneus longus and brevis. A heavy fibrofatty heel pad firmly adheres to the calcaneus and skin and provides protection during heel strike (see Fig. 118-1).
Because the strong ankle extensor attachments are divided in the performance of midfoot and hindfoot amputation, the Achilles tendon exerts unopposed plantar flexor forces on the residual foot. The Achilles tendon may require division or lengthening to prevent the development of an equinus deformity.
Lisfranc’s and Chopart’s Amputations
Lisfranc’s.
Lisfranc first described this amputation in 1815. The incision results in a long plantar flap (see Figs. 118-5 and 118-6). Tendons and synovial sheaths are divided at the level of the skin incision. The first, third, fourth, and fifth tarsometatarsal joints are disarticulated. The second metatarsal is divided 1 to 2 cm distal to the medial cuneiform.28 To reduce the risk of development of equinovarus deformity in the patient, Sanders has recommended a modification of the Lisfranc amputation that preserves the base of the fifth metatarsal and the insertion of the peroneus brevis.25 The Achilles tendon is released by either transection or Z-plasty.29,30 The plantar fascia on the flap is approximated to the dorsal periosteum with absorbable sutures. The skin is approximated with interrupted monofilament sutures or staples.
Chopart’s.
Chopart described this amputation in 1814. It involves a long plantar flap similar to that used in the Lisfranc amputation (see Figs. 118-5 and 118-6). This amputation is performed through the talocalcaneonavicular joint and the calcaneocuboid joint. To prevent equinovarus deformity resulting from unopposed plantar flexion, an Achilles tenectomy is recommended. The extensor hallucis longus and the tibialis anterior tendons may be reattached to the talar neck. The extensor digitorum longus may be reattached to the calcaneus.28 The wound is irrigated and closed in layers.
Postoperative Considerations.
Because the Lisfranc and Chopart amputations result in a dramatic alteration in normal foot biomechanics, their durability may be limited. A short-leg plaster cast is used over the sterile dressings. The cast must be molded to ensure that the talus is slightly dorsiflexed in relation to the tibia and that the calcaneal tuberosity is parallel to the long axis of the tibia. The cast is changed weekly to check wound healing. Weight bearing is allowed after 6 weeks. Patients with a Lisfranc amputation need little more than a shoe filler with an ankle lace-up shoe. Patients with a Chopart amputation need a custom-fitted ankle-foot orthosis with a filler to hold the shoe adequately.
Syme’s Amputation
Syme first described this amputation in 1843. The anterior incision extends across the ankle just distal to the tip of each malleolus (see Figs. 118-5 and 118-7). The posterior incision extends from the malleoli vertically down and across the sole of the foot. The extensor tendons are divided at the level of the skin incision. The dorsalis pedis artery is ligated and divided. The ankle joint capsule is incised while plantar flexing the foot. The medial and lateral ankle ligaments are divided. The tendons of the posterior tibialis and flexor hallucis longus are transected, taking care to avoid injury to the posterior tibial artery. The heel fat pad is carefully dissected by staying close to the calcaneus to avoid buttonholing. The ankle joint is disarticulated, and the specimen is passed off the table. In a one-stage amputation, the malleoli are divided with a saw at the level of the articular surface of the tibia, and the width is reduced by vertical bone excision. Holes are drilled in the medial, anterior, and lateral parts of the distal tibia and fibula to secure the heel pad directly under the tibia. In a two-stage Syme amputation, the wound is closed by suturing the heel flap to the dorsal fascia. Six weeks later, the malleoli are removed through separate vertical incisions. (See references 31 and 32 for a detailed description of midfoot amputations.)
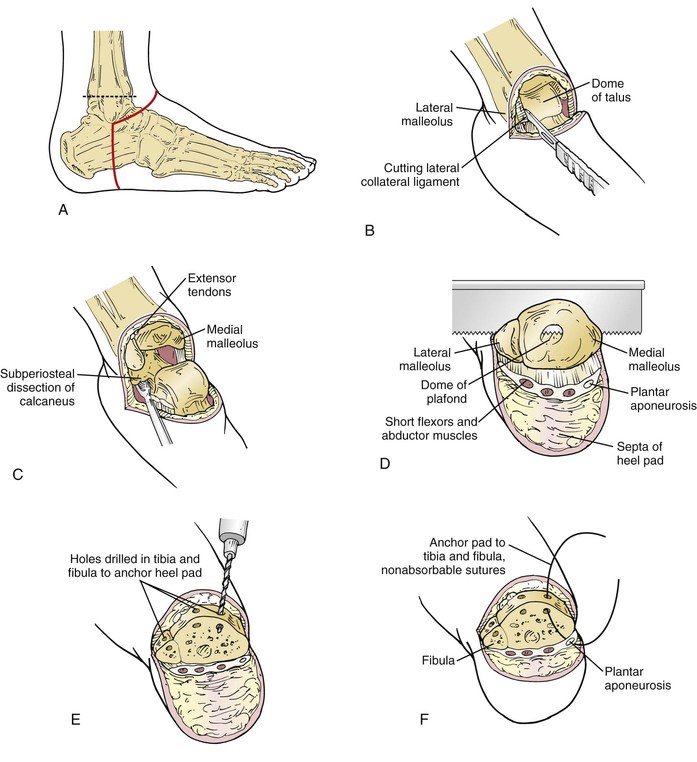
Figure 118-7 Syme’s amputation. A, Skin incision and bone transection level. B, Exposure of the ankle and division of the ligaments. C, Soft tissue dissection from the calcaneus. D, Division of the tibia and fibula. E, Holes drilled in the anterior aspect of the tibia and fibula. F, Fascia lining the heel pad sutured to the bone.
Postoperative Considerations.
The chief advantage of the Syme amputation is the preservation of limb length, which may obviate the need for a prosthesis during brief periods of weight bearing, such as during transfer from bed to wheelchair. The disadvantages include the slight leg-length discrepancy, which may lead to biomechanical side effects in more proximal or contralateral joints. Further, the prosthesis is typically bulky at the ankle and less cosmetically appealing than a conventional below-knee prosthesis, which can be designed with a near-normal ankle profile. In addition, because it is an end weight-bearing stump, the heel fat pad may be unstable and can migrate medially, exposing the distal tibia to excessive force. Thus, the Syme amputation has its greatest utility in young, healthy trauma patients with extensive forefoot injuries who are highly motivated to avoid transtibial amputation. We consider the presence of diabetic neuropathy a specific contraindication to Syme’s amputation because of the inability to protect the stump from injuries such as scalding and pressure.
Transtibial (Below-Knee) Amputation
Anatomy
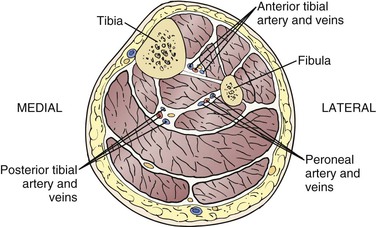
There are four muscle compartments in the leg (Fig. 118-8). The anterior compartment contains the tibialis anterior, extensor hallucis longus, and extensor digitorum longus muscles and the deep peroneal nerve. The lateral compartment contains the peroneus longus and brevis muscles and the superficial peroneal nerve. The posterior compartment is subdivided into superficial and deep parts. The superficial posterior group consists of the gastrocnemius and soleus muscles. The deep posterior compartment contains the tibialis posterior, flexor digitorum longus, and flexor hallucis longus muscles. The tibial nerve is adjacent to the posterior tibial artery. The tibia and fibula are connected distally by a fibrous inferior tibiofibular joint that is sacrificed in conventional BKA. The skin of the leg is supplied by fasciocutaneous perforating arteries that arise from the posterior tibial, anterior tibial, sural, saphenous, and peroneal arteries (Fig. 118-9). Of note, the sural artery arises from the popliteal artery proximal to the tibial trifurcation and supplies the gastrocnemius muscle and the posterior skin. The saphenous artery accompanies the saphenous vein and nerve below the knee and supplies the skin of the anteromedial leg, along with perforating branches of the posterior tibial artery.33 To preserve adequate blood supply to the skin via these fasciocutaneous perforators, the skin and subcutaneous tissues should not be separated from the underlying fascia.
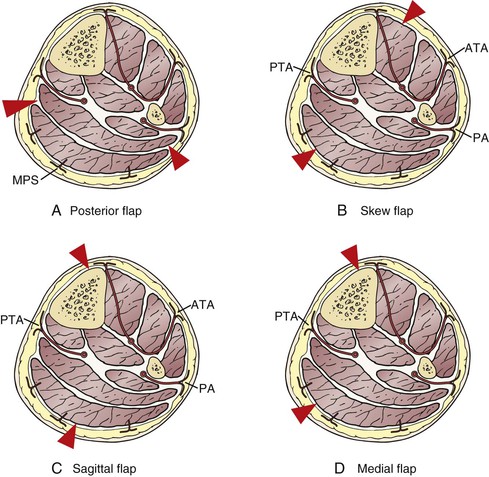
Figure 118-9 Blood supply to the skin at the level of transtibial amputation. Note the location of skin incisions (red arrowheads). A, Posterior flap. B, Skew flap. C, Sagittal flap. D, Medial flap. ATA, Anterior tibial artery; MPS, musculocutaneous perforators from the sural artery; PA, peroneal artery; PTA, posterior tibial artery.
Technique
Five types of flaps are used in transtibial amputation techniques (see Fig. 118-9).
Posterior Flap.
The most common technique employs a long posterior flap as described by Burgess et al.34–36 Optimally, the tibia should be divided 10 to 12 cm (three or four fingerbreadths) distal to the tibial tuberosity; however, we have observed functional stumps with as little as 5 cm of residual tibia (Fig. 118-10). The anterior incision extends from one half to two thirds of the leg’s circumference. A thicker posterior flap results in more prominent dog-ears but may be better vascularized. The length of the posterior flap is approximately one third the circumference of the leg and should be gently curved to reduce dog-ears. After venous exsanguination and application of a pneumatic thigh-high tourniquet, the skin and fascia are incised together, beginning with the transverse component and then extending to complete the posterior flap. The anterior and lateral compartment muscles are divided. The periosteum of the tibia is incised circumferentially, and the tibia is divided using a power saw perpendicular to the long axis of the bone. The anterior lip of the tibia is beveled to eliminate sharp edges that may protrude through thin skin. The fibula is divided with a saw no more than 1 to 2 cm proximal to the tibia. Excessive resection of the fibula results in a conical stump that is difficult to fit with a prosthesis. The posterior flap is completed by dividing the residual posterior compartment musculature at a plane just deep to the tibia and fibula with a long amputation knife. Major vascular bundles are suture ligated. The tourniquet is released, and complete hemostasis is obtained. The tibial and peroneal nerves are sharply divided and allowed to retract to avoid the formation of a neuroma. If necessary, the posterior flap can be trimmed, but excessive attempts to eliminate dog-ears should be avoided because the stump will remodel with time. Some surgeons prefer to advance the posterior flap 3 to 4 cm proximal to the tibial osteotomy.37 If the posterior flap is too bulky to allow tension-free closure, the soleus muscle can be excised at the level of the tibial osteotomy, taking care to preserve the gastrocnemius muscle and fascia. The sural nerve is identified in the subcutaneous tissue in the middle of the posterior flap and divided 5 or 6 cm proximal to the skin edge to prevent neuroma formation. The wound is irrigated to remove bone dust. The deep fascia is approximated with interrupted absorbable sutures, taking care to completely cover the tibia without tension. The skin is closed with staples or monofilament suture.
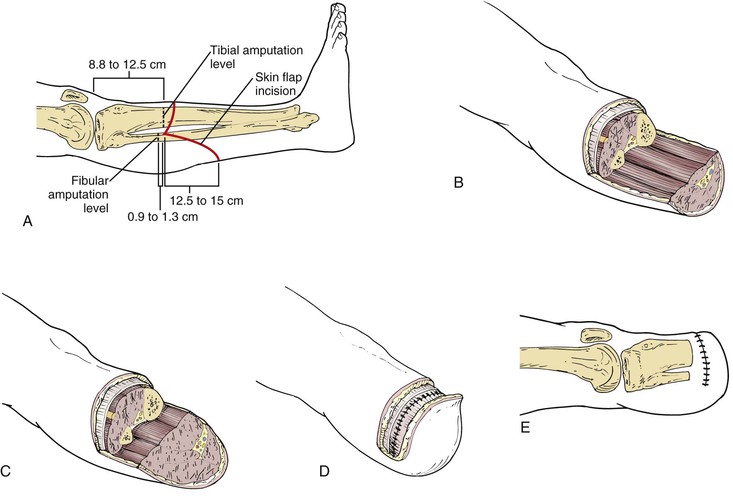
Figure 118-10 Transtibial amputation. A, Marking the skin incisions. B, Fashioning the flaps after bone transection. C, The soleus muscle is tailored to create a proper flap. D, The posterior deep fascia is sutured to the anterior deep fascia and periosteum. E, Closure of skin flaps.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree