The presence of clinical peripheral arterial disease (PAD) is associated with an increased risk for adverse cardiovascular outcomes in patients with coronary artery disease. However, there are few data regarding the impact of the presence and degree of the subclinical PAD on outcomes in patients with coronary artery disease. The aim of this study was to assess prospectively the grade of subclinical PAD in the setting of patients who underwent primary percutaneous coronary intervention for the prediction of intermediate- and long-term clinical outcomes. A total of 971 consecutive patients without histories of clinical PAD who under went primary percutaneous coronary intervention for ST-segment elevation myocardial infarction were included in a prospective follow-up. Subclinical PAD severity was blindly assessed on the basis of an ultrasound arterial morphologic classification defined with the assessment of wall carotid and femoral artery bifurcations. This classification included 4 increasing classes of subclinical carotid and femoral arterial wall lesions, and the total group was divided accordingly. Death and major cardiovascular and cerebrovascular events were evaluated. During a median follow-up period of 40 months, a total of 109 patients (11.2%) died, 9 (2.8%) in class I, 12 (3.1%) in class II, 37 (23.7%) in class III, and 51 (49.0%) in class IV (p <0.001). On multivariate analysis, mortality in class IV was sevenfold higher (hazard ratio [HR] 7.34, 95% confidence interval [CI] 3.3 to 16.33, p <0.001) compared to class I and was also increased in class III (HR 5.38, 95% CI 2.42 to 11.92, p <0.001). Similar results were obtained for major adverse cardiovascular and cerebrovascular events in class IV (HR 7.50, 95% confidence interval 5.36 to 10.50, p <0.0001), class III (HR 6.44, 95% CI 4.45 to 9.32, p <0.001), and class II (HR 1.73, 95% CI 1.23 to 2.43, p = 0.002). In conclusion, ultrasound arterial morphologic classification may be applied in patients with ST-segment elevation myocardial infarctions who undergo primary percutaneous coronary intervention and can stratify patients for poor clinical outcomes during long-term follow-up.
The presence of clinical peripheral arterial disease (PAD) in patients with coronary artery disease (CAD) who undergo percutaneous coronary intervention (PCI) is associated with short-term and midterm morbidity and mortality. However, there are few data regarding the impact on the presence and degree of subclinical PAD on outcomes in patients with CAD, especially those who undergo PCI for ST-segment elevation myocardial infarction (STEMI). Ultrasound arterial morphologic classification (UAMC) of carotid and femoral wall changes detected by high-resolution ultrasound is a simple technique to evaluate the severity of subclinical atherosclerosis. Using this method, asymptomatic subjects can be separated into subgroups of different risk for cardiovascular events according the degree and extent of subclinical atherosclerotic lesions. We aimed to assess prospectively the grade of subclinical PAD using high-resolution ultrasound of the carotid and femoral arterial wall in the setting of patients who underwent primary PCI for the prediction of short- and long-term clinical outcomes.
Methods
From 2002 to 2010, we enrolled all consecutive patients admitted to our coronary care unit for STEMI who were treated with primary PCI. According to our institute protocol, patients were included if they presented <12 hours after symptom onset (characteristic chest pain lasting ≥30 minutes, not responsive to nitrates, with electrocardiographic ST-segment elevation ≥0.2 mV in ≥2 contiguous leads, or development of new left bundle branch block). Patients receiving long-term peritoneal or hemodialysis treatment and patients with cardiogenic shock were excluded. Furthermore, patients were excluded if they had histories and/or clinical symptoms consistent with PAD or histories of peripheral arterial surgery or angioplasty and if they were wheelchair bound or were unwilling or unable to provide informed consent. All participants provided written informed consent.
Primary PCI was performed by a 24-hour on-call interventional team, according to standard clinical practice. All patients received a 300-mg loading dose of clopidogrel, in combination with acetylsalicylic acid 100 mg. After sheath insertion, a heparin bolus at a dose of 70 U/kg, followed by an additional bolus during the procedure to maintain activated clotting time >300 seconds if deemed necessary, and an intravenous bolus and an infusion of platelet glycoprotein IIb/IIIa receptor inhibitors (abciximab) were administered. Supportive pharmacologic therapy was left to the discretion of the interventional and coronary care unit cardiologists, according to our institution’s clinical protocols and international guidelines. Dual-antiplatelet therapy (aspirin 100 mg/day and clopidogrel 75 mg/day) was administrated in all patients for ≥1 month after bare-metal stent insertion and 6 to 12 months after drug-eluting stents implantation. All patients were advised to maintain aspirin (100 mg/day) lifelong.
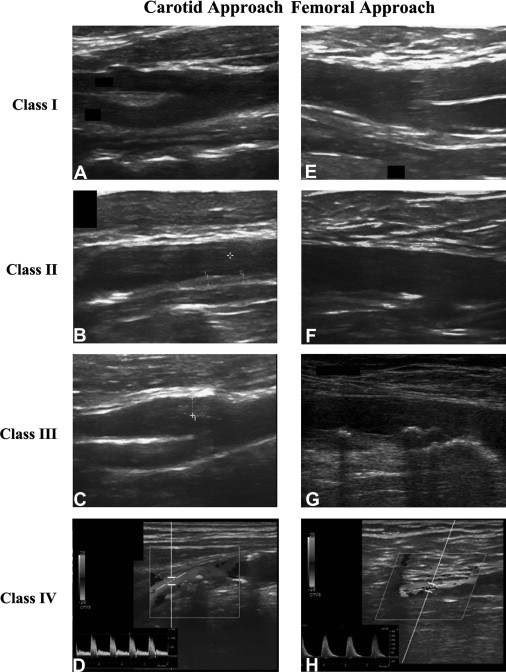
Echocardiographic evaluations were performed in all patients before the procedure to assess wall motion abnormalities and ejection fractions. Serum creatinine concentration was measured at the time of admission (just before primary PCI), every day for the following 3 days, and at discharge from the coronary care unit. Estimated glomerular filtration rate was calculated by applying the Modification of Diet in Renal Disease (MDRD) formula. All patients satisfying inclusion criteria underwent carotid and femoral UAMC assessment during hospitalization or <2 weeks after PCI. The carotid and femoral arterial bifurcations were studied using a Vivid e ultrasound system, using a high-resolution 8L-RS, 12.0-MHz linear transducer (GE Healthcare, Wauwatosa, Wisconsin). UAMC assessment was performed using the method originally described by Belcaro et al. The carotid and femoral bifurcations were localized by a transverse scan. The probe was rotated 90° to obtain and record the longitudinal image of both the anterior and posterior walls, with the latter being used for evaluation. Both arteries were evaluated for a length of 3 cm (1.5 cm proximally and distally to the flow divider). By this technique, the 3 ultrasonic vessel wall layers (intima-media, adventitia, and periadventitia) were clearly visible. Technical ultrasound parameters (dynamic range, depth range, power, reject, edge, grayscale, and smooth) were maintained constant. All ultrasound images were recorded on the hard drive in the ultrasound system and then stored on a magneto-optical disc for later off-line analysis. The initial classification included 5 classes corresponding to 5 scores, ranging from 0 to 8 for each artery. In the present study, the previously described classes I (normal wall) and II (wall initial disruption) were merged into 1 class, because it was not possible to differentiate class I from II on the basis of echocardiographic morphology and/or association with the subsequent incidence of cardiovascular events. Therefore, only 4 classes were used (class I, normal or initial wall disruption; class II, wall thickening; class III, nonstenosing plaques; and class IV, stenosing plaques) corresponding to 4 scores ranging from 2 to 8 for each artery (total score ranging from 8 to 32 in each; Table 1 , Figure 1 ). Each subject was placed in a class (I through IV) according to the worst artery and received a score obtained by adding the scores of the 4 arteries studied. Ultrasound, was performed blinded to the report of angiographic coronary anatomy or the PCI procedure. The operators performing the scans were 3 physicians with ≥2 years of clinical experience and practice with B-mode, duplex and color duplex scanning, with the assistance of a sonographer with extensive experience.
| Class | Ultrasound Morphology | Score ∗ |
|---|---|---|
| I | Normal: 3 ultrasonic layers (intima-media, adventitia, and periadventitia) clearly separated; no disruption of lumen-intima interface for ≥3.0 cm, and/or initial alterations (lumen-intima interface disruption at intervals of <0.5 cm) | 2 |
| II | Intima-media granulation: granular echogenicity of deep, normally anechoic intimal-medial layer and/or increased intima-media thickness (>1 mm) | 4 |
| III | Plaque without hemodynamic disturbance † : localized wall thickening and increased density involving all ultrasonic layers; intima-media thickness >2 mm | 6 |
| IV | Stenotic plaque: as in class III, but with hemodynamic stenosis on duplex scanning (sample volume in the center of the lumen), indicating stenosis >50% ‡ | 8 |
∗ The score is relative to 1 artery. The patient’s score is the sum of the scores of all 4 arteries.
† Hemodynamic disturbance is defined as moderate spectral broadening (down stroke of systole); systolic window present; diastolic window reduced and/or absent. Ratio a − b/a <0.5, where a is the ‡ peak frequency >4 kHz and spectral broadening throughout systole; no systolic window. Ratio a − b/a <5 peak systolic velocity and b, the first peak end-systolic velocity.
Intraobserver variability of ultrasound optical disc recording and class attribution was assessed in 40 subjects (10 from each UAMC class, 4 arteries per patient) on 2 different occasions within 2 weeks. The 2 recordings and class attribution were performed by the same physician, who was unaware of the result of the first examination. The coefficient of variation was on average 5% (2 arteries not correctly classified in the lower or higher class out of 40 arteries in each class). Interobserver variability was lower than intraobserver variability. The error in class attribution in 40 recordings (40 patients) blindly read and classified by 2 different observers, unaware of the identities of the subjects under evaluation, was 2.50% (4 classification discrepancies of 1 arterial morphology class out of 160 studied recordings). These results are in agreement with previous estimates.
All patients were followed in the outpatient clinic of the Cardiology Unit at Modena University Hospital, with clinical examinations at 1, 6, and 12 months and yearly thereafter. In addition, telephone interviews of patients or their general practitioners were conducted for patients who failed to present during follow-up in the outpatient clinic. Events recorded in this analysis were cardiac versus noncardiac death, occurrence of new Q-wave and non-Q-wave myocardial infarction, major stroke, need for target lesion revascularization, and coronary artery bypass graft surgery performed after the emergency procedure.
Chronic renal insufficiency was defined as estimated glomerular filtration rate <60 ml/min/1.73 m 2 , according to the MDRD equation. Cardiogenic shock was defined as systolic blood pressure <90 mm Hg lasting ≥1 hour, not responsive to fluid replacement or loading, and/or heart rate correction believed to be secondary to cardiac dysfunction and associated with ≥1 of the following signs of systemic hypoperfusion: cool, clammy skin, oliguria, altered sensorium, and cardiac index ≤2.2 L/min/m 2 . Anemia was defined using World Health Organization criteria, such as a baseline hemoglobin value <13 g/dl for men and <12 g/dl for women. Heart failure was defined as New York Heart Association functional classification III or IV and/or a history of pulmonary edema. Diabetes mellitus was defined using the criteria of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, such as fasting plasma glucose ≥7.0 mmol/L (126 mg/dl) or 2-hour plasma glucose ≥11.1 mmol/L (200 mg/dl). Dyslipidemia was operationalized as pathologic lipid levels according to the guidelines of the National Cholesterol Education Program Adult Treatment Panel III and/or treatment with lipid-lowering drugs. Hypertension was defined as elevated blood pressure according to guidelines of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and/or ongoing antihypertensive drugs. A lifetime history of >100 cigarettes smoked was considered a positive smoking history. Multivessel coronary disease was defined as the presence of ≥70% stenosis of the luminal diameter in a major epicardial artery, with ≥50% stenosis in a second major epicardial vessel (2-vessel disease) or in both of the other epicardial vessels (3-vessel disease). Anatomic success of PCI with stenting was defined as achievement of a minimal diameter stenosis <20% as visually assessed by angiography and maintenance of Thrombolysis In Myocardial Infarction (TIMI) flow grade 3. Anatomic success of PCI without stenting was defined as stenosis diameter reduction >20% with residual stenosis <50%. No-reflow phenomenon was defined as TIMI grade 0, 1, or 2 flow after PCI in the absence of mechanical obstruction. Major stroke was defined as any new neurologic deficit of vascular origin with a related disability resulting in a score ≥2 on the modified Rankin scale at 1 month after clinical onset. Stroke diagnosis and disability assessments were made by an independent neurologist. The primary end point of the study was the rate of major adverse cardiovascular and cerebrovascular events (MACCEs), defined as the composite of death, stroke, nonfatal acute myocardial infarction, and repeated revascularization of target vessel by PCI or coronary artery bypass graft surgery during the hospital stay or during follow-up. Table 1 lists the criteria for the UAMC calculation.
On the basis of the UAMC assessment, the population was divided into 4 subclinical atherosclerosis classes (class I, normal or initial wall disruption; class II, wall thickening; class III, nonstenosing plaques; and class IV, stenosing plaques). Continuous data are reported as mean ± SD unless otherwise specified. Categorical data are presented as absolute values and percentages. Comparison of continuous variables was performed by using analysis of variance. Fisher’s exact chi-square test was used for comparisons of categorical variables. Survival and the cumulative incidence of events were represented using Kaplan-Meier curves. The significance of the curves was tested using the log-rank test. For MACCE criteria, in the case of multiple events, the first episode was taken as the standard. During follow-up, variables predictive of events were calculated through univariate and multivariate analysis by Cox regression and presented with hazard ratios and 95% confidence intervals. Covariates were selected on the basis of previous knowledge of factors assessed in the emergency department that are known predictors of mortality. Covariates were kept in the models, regardless of significance, and the stratified Cox model was adjusted for demographics (age), co-morbidities (anemia, chronic kidney disease, and diabetes), and presenting and periprocedural characteristics (Killip class and the ejection fraction). The accuracy of the risk score was tested using receiver-operating characteristic analysis and evaluating the area under the curve. All tests were 2 tailed, and probability was considered to be statistically significant at p <0.05. All analyses were performed using Stata/SE version 10.0 for Windows (StataCorp LP, College Station, Texas).
Results
Among a total of 1,168 patients with STEMIs, 197 were excluded (43 with histories and/or clinical symptoms consistent with PAD, 26 with histories of peripheral arterial surgery or angioplasty, 62 with cardiogenic shock, 18 receiving chronic peritoneal or hemodialysis treatment, and 43 for inability to perform UAMC assessment, e.g., for early return after angioplasty to the peripheral hospital of provenance). Hence, a total of 971 patients (743 men, 208 women; mean age 64 ± 13 years) were included in this study. Follow-up was completed in 971 patients (100%) at 1 year. The median follow-up period was 40 ± 18.2 months, with up to 92.5 months of follow-up. Table 2 list the baseline clinical and procedural characteristics of the UAMC classes of patients who underwent primary PCI. With increasing subclinical PAD severity assessed using UAMC classes, patients were significantly older, more frequently had chronic kidney disease, and were in Killip class II or III at presentation. There were no significant difference with increasing class with regard to the percentages of men, smokers, patients with diabetes, patients with hypertension, patients with family histories of cardiovascular disease, and patients with history of coronary revascularization. Otherwise, stratifying patients into low (classes I and II) versus high (classes III and IV) subclinical atherosclerosis degree, a significant difference was found regarding diabetes mellitus (p = 0.047). Patients in higher UAMC classes had numerically more coronary multivessel disease, lesions involving the left main coronary artery, and a lower mean ejection fraction, but the difference did not reach statistical significance. Furthermore, there were no significant differences in relation to the procedural characteristics of PCI or to medical treatment at hospital discharge. However, there was a trend toward significance with regard to the use of higher amounts of contrast agent in the patients in greater UAMC classes.
| Variable | Overall (n = 971) | Carotid and Femoral UAMC Groups | p Value | |||
|---|---|---|---|---|---|---|
| I (n = 322 [33.1%]) | II (n = 389 [40.1%]) | III (n = 156 [16.1%]) | IV (n = 104 [10.7%]) | |||
| Age (yrs) | 63.7 ± 13.0 | 62.7 ± 12.8 | 63.3 ± 13.5 | 62.4 ± 11.7 | 71.1 ± 12.5 | <0.001 |
| Men | 743 (76.6%) | 339 (77.6) | 164 (72.6) | 159 (80.3) | 81 (74.3) | 0.249 |
| Body surface area (m 2 ) | 1.86 ± 0.1 | 1.86 ± 0.18 | 1.86 ± 0.17 | 1.85 ± 0.20 | 1.84 ± 0.17 | 0.301 |
| Weight (kg) | 76.9 ± 13.6 | 74.7 ± 12.9 | 77.9 ± 13.6 | 77.0 ± 12.6 | 76.5 ± 14.5 | 0.027 |
| Diabetes mellitus | 143 (14.7%) | 40 (12.4) | 55 (14.1) | 31 (19.9) | 17 (16.3) | 0.174 |
| Smokers | 339 (34.9%) | 166 (37.9) | 78 (34.5) | 65 (32.8) | 30 (27.5) | 0.232 |
| Dyslipidemia | 173 (17.8%) | 57 (17.7) | 70 (18.0) | 26 (16.7) | 20 (19.2) | 0.961 |
| Hypertension | 431 (44.3%) | 136 (42.2) | 169 (43.4) | 75 (48.0) | 50 (48.6) | 0.433 |
| Previous PCI | 92 (9.5%) | 31 (9.6) | 29 (7.5) | 18 (11.5) | 14 (13.5) | 0.207 |
| Previous coronary artery bypass graft surgery | 34 (3.5%) | 12 (2.8) | 9 (3.1) | 7 (4.5) | 6 (5.8) | 0.439 |
| Presenting characteristics | ||||||
| Systolic blood pressures (mm Hg) | 129.8 ± 27.0 | 131.9 ± 26.3 | 131.2 ± 26.6 | 125.8 ± 28.2 | 124.2 ± 2.3 | 0.014 |
| Anterior STEMI | 510 (52.5%) | 161 (50.0) | 221 (56.8) | 78 (48.1) | 50 (50.0) | 0.178 |
| Killip class II | 175 (18.0%) | 41 (12.7) | 55 (14.1) | 40 (34.6) | 39 (37.5) | <0.001 |
| Killip class III | 42 (4.3%) | 10.0 (3.1) | 11 (2.8) | 13 (8.3) | 8 (7.7) | <0.001 |
| Multivessel CAD | 153 (15.8%) | 37 (11.5) | 70 (14.7) | 23 (18.0) | 23 (22.1) | 0.027 |
| Left main CAD | 33 (3.4%) | 8 (2.5) | 15 (3.9) | 3 (1.9) | 7 (6.7) | 0.132 |
| Chronic kidney disease | 200 (20.6%) | 56 (17.4) | 65 (16.7) | 39 (25.0) | 40 (38.5) | <0.001 |
| Anemia | 196 (20.2%) | 54 (16.8) | 73 (18.8) | 34 (21.8) | 35 (33.7) | 0.002 |
| Preprocedural creatinine (mg/dl) | 1.1 ± 0.77 | 1.1 ± 0.8 | 1.0 ± 0.5 | 1.1 ± 0.3 | 1.5 ± 1.4 | <0.001 |
| Preprocedural left ventricular ejection fraction (%) | 45.3 ± 10.0 | 46.1 ± 9.2 | 45.3 ± 10.3 | 45.2 ± 9.9 | 42.7 ± 11.1 | 0.044 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 79.5 ± 25.6 | 82.0 ± 25.9 | 81.9 ± 24.3 | 77.1 ± 24.0 | 66.3 ± 27.8 | <0.001 |
| Procedural characteristics | ||||||
| Symptom-to-balloon time (min) | 236.9 ± 136.5 | 235.9 ± 130.2 | 237.7 ± 155.5 | 242.5 ± 130.3 | 228.3 ± 131.7 | 0.861 |
| Anatomic success of PCI | 914 (94.1%) | 301 (93.5) | 373 (95.9) | 146 (93.6) | 97 (93.2) | 0.346 |
| No-reflow phenomenon | 39 (4.0%) | 8 (2.5) | 16 (4.1) | 11 (7.1) | 4 (3.8) | 0.127 |
| Rate of stenting | 902 (92.8%) | 302 (93.7) | 360 (92.54) | 135 (86.5) | 93 (89.4) | 0.299 |
| Bare-metal stent | 786 (80.9%) | 267 (82.9) | 310 (79.69) | 117 (75.0) | 82 (78.8) | 0.321 |
| Drug-eluting stent | 116 (11.9%) | 35 (10.8) | 50 (12.8) | 18 (11.5) | 11 (10.5) | 0.231 |
| Glycoprotein IIb/IIIa receptor inhibitors | 826 (85.1%) | 275 (85.4) | 324 (83.29) | 136 (87.1) | 91 (87.5) | 0.959 |
| Mean quantity of contrast agent (ml) | 215.0 ± 88.0 | 205.8 ± 80.8 | 211.7 ± 89.2 | 230.2 ± 93.0 | 232.4 ± 92.9 | 0.006 |
| Medical treatments at hospital discharge | ||||||
| β blockers | 772 (79.5%) | 248 (77.1) | 304 (78.2) | 125 (80.3) | 86 (82.33) | 0.564 |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 637 (65.6%) | 210 (66.2) | 265 (68.2) | 102 (65.3) | 65 (62.7) | 0.376 |
| Statins | 833 (85.8%) | 271 (84.2) | 331 (85.2) | 135 (86.7) | 91 (87.2) | 0.065 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


