Patients with acute coronary syndrome (ACS) and nonobstructive coronary artery disease (CAD) have a substantial risk of subsequent coronary events within 1 year. The aim of the present study was to evaluate the prevalence, long-term outcomes, and adherence to oral antiplatelet therapy in patients with ACS and nonobstructive CAD compared with patients with ACS and obstructive CAD who had undergone percutaneous coronary intervention. Nonobstructive CAD was defined as an angiographic finding of <50% diameter stenosis in any major epicardial artery. These patients were further stratified into 2 groups: those with normal coronary arteries (0% angiographic stenosis) and those with mild CAD (0% to 50% angiographic stenosis). Major adverse cardiac events, defined as death, myocardial infarction, ACS leading to hospitalization, and nonfatal stroke, were recorded and compared with a historical control group of patients with ACS and obstructive CAD who had undergone percutaneous coronary intervention. Of 2,438 consecutive patients with ACS undergoing coronary angiography, 318 (13%) had nonobstructive CAD. Of the 318 with nonobstructive CAD, 160 had normal coronary arteries and 158 had mild CAD. Patients with obstructive CAD had experienced greater rates of major adverse cardiac events at 26 ± 16 months (16.6% vs 9.1%, p = 0.001), driven by a greater rate of myocardial infarction compared with those without (5.3% vs 0%, p <0.001). However, the rate of death, ACS leading to hospitalization, and stroke was similar. After adjusting for baseline characteristics, no difference was found in the risk of major adverse cardiac events across the groups. Only 50% of patients with nonobstructive CAD were prescribed dual antiplatelet therapy. In conclusion, patients with ACS and nonobstructive CAD remain at high risk of long-term recurrent ischemic events.
Approximately 25% of female and 10% of male patients presenting with acute coronary syndrome (ACS) have nonobstructive coronary artery disease (CAD), including normal coronary arteries (NCA) or mild CAD (MCAD), defined as the presence of coronary stenoses <50%. Importantly, the prognosis of these patients might not be as benign as commonly assumed. Data from the Thrombolysis In Myocardial Infarction 11B, 16, and 22 trials have suggested that 12% of patients presenting with ACS and nonobstructive CAD will have experienced an adverse cardiac event within 12 months, with a 2% incidence of combined death or myocardial infarction. However, the prevalence and long-term prognosis of nonobstructive CAD in unselected real-world patients with ACS have been poorly investigated. In particular, comparative studies of the long-term outcomes of patients with nonobstructive CAD receiving optimal medical therapy versus patients with obstructive CAD referred for percutaneous coronary intervention are lacking. In addition, patients with nonobstructive CAD have been less likely to receive evidence-based medication at discharge compared with patients with obstructive CAD ; however, data regarding the long-term adherence to oral antiplatelet therapy by these patients are lacking. The aim of the present study was to evaluate the prevalence and long-term outcomes (ischemic events and bleeding) and long-term adherence to oral antiplatelet therapy of unselected patients with ACS and nonobstructive CAD compared with patients with ACS and obstructive CAD who had undergone percutaneous coronary intervention.
Methods
Of 2,438 consecutive patients admitted with a diagnosis of ACS at 4 Italian centers (Ospedali Riuniti di Bergamo; Ospedale Carlo Poma, Mantova; Ospedaledella Misericordia, Grosseto; and Ospedale Galliera, Genova, Italy) from January 2009 to December 2009, 318 (13%) had NCA (0% angiographic stenosis) or MCAD (>0% and <50% angiographic stenosis) found on the coronary angiogram. This cohort was compared with a historical control group of 888 patients presenting with ACS and obstructive CAD who had undergone percutaneous coronary intervention in 2010 at the same institutions. Oral antiplatelet drugs and glycoprotein IIb/IIIa inhibitors were administered at the physician’s discretion.
The patients were followed up for 26 ± 16 months by telephone interview or outpatient clinical visits. The occurrence of major adverse cardiac events (MACE) and the adherence to dual oral antiplatelet therapy with aspirin and a thienopyridine (ticlopidine or clopidogrel) were assessed. Novel P2Y 12 receptor antagonists (prasugrel or ticagrelor) were not in clinical use at the time of this registry. Whenever adverse cardiac events and/or major bleeding events occurred, the patients’ charts were reviewed.
ACS was defined according to the American College of Cardiology/American Heart Association criteria. MACE was defined as the composite of death, myocardial infarction, ACS leading to hospitalization, and nonfatal stroke. Death was considered as cardiac in origin unless obvious noncardiac causes could be identified. Sudden death was defined as unexplained death in previously stable patients. Myocardial infarction was diagnosed if any troponin elevation with symptoms suggestive of ACS was detected. The presence of new pathologic Q waves on the electrocardiogram was also considered myocardial infarction. Within 1 week of the index event, only Q-wave myocardial infarction was adjudicated as myocardial infarction. Myocardial infarction occurring during percutaneous coronary intervention was defined by a new Q wave lasting >0.03 second in 2 contiguous electrocardiographic leads or elevations in creatine kinase and the MB fraction of creatine kinase, including an increase in the creatine kinase-MB level that was ≥3 times the local upper limit of the normal range. Myocardial infarction was also diagnosed if biomarkers elevated at baseline had increased an additional 50% greater than at baseline after percutaneous coronary intervention. The diagnosis of destabilizing cardiac symptoms was defined as the occurrence of ischemic symptoms requiring hospitalization without any biochemical evidence of myocardial necrosis. Stroke was defined as an ischemic cerebral infarction caused by an embolic or thrombotic occlusion of a major intracranial artery. Bleeding complications were classified as major or minor according to the Thrombolysis In Myocardial Infarction criteria. Major bleeding included any intracranial bleeding or any bleeding associated with clinically overt signs associated with a decrease in hemoglobin >5 g/dl. Minor bleeding was defined as any clinically overt sign of bleeding (including observation using imaging studies) associated with a decrease in hemoglobin ≥3 and ≤5 g/dl.
Continuous variables are presented as the mean ± SD or median and interquartile range and were compared using the Student unpaired t or Mann-Whitney rank sum tests, as appropriate. Categorical variables are presented as counts and percentages and were compared using the chi-square test, when appropriate (expected frequency >5). Otherwise, the Fisher exact test was used. Multivariate logistic regression models were applied to adjust for baseline confounders. Variables considered for multivariate adjustment included those significant on univariate analysis and/or deemed of clinical importance (n = total MACE/10). The results are reported as odds ratios and 95% confidence intervals.
Results
The baseline demographics, clinical characteristics, and procedural features of the patients with NCA, MCAD, and obstructive CAD are listed in Table 1 . A total of 160 and 158 patients had NCA and MCAD, respectively. The NCA patients were younger (63 ± 16 vs 68 ± 12 years, p = 0.007) and were more frequently women (61.2% vs 47.5%, p = 0.014) compared with the patients with MCAD. Among the patients with MCAD, nonobstructive lesions were located in the left main, left anterior descending, left circumflex, and right coronary artery in 4%, 59%, 43%, and 37%, respectively. The patients with NCA/MCAD combined were older than the patients with obstructive CAD (66 ± 15 vs 63 ± 12 years, p = 0.008) and were more likely to be women (64.4% vs 21.5%, p <0.001). However, the patients with obstructive CAD more often presented with a history of previous myocardial infarction (22.6% vs 6%, p <0.001) and previous revascularization.
| Variable | NCA/MCAD (A+B; n = 318) | NCA (A; n = 160) | MCAD (B; n = 158) | Obstructive CAD (C; n = 888) | p Value | ||
|---|---|---|---|---|---|---|---|
| A vs B | A vs B vs C | A+B vs C | |||||
| Age (yrs) | 66 ± 15 | 63 ± 16 | 68 ± 12 | 63 ± 12 | 0.007 | <0.001 | 0.008 |
| Men | 145 (35.6) | 62 (38.8) | 83 (52.5) | 697 (78.5) | 0.014 | <0.001 | <0.001 |
| BMI (kg/m 2 ) | 27 ± 5 | 26 ± 5 | 27 ± 5 | 27 ± 4 | 0.57 | 0.79 | 0.94 |
| Smoking status | 0.72 | <0.001 | <0.001 | ||||
| Nonsmoker | 239 (75.2) | 120 (75.0) | 119 (75.3) | 459 (51.7) | |||
| Active | 73 (23.0) | 36 (22.5) | 37 (23.6) | 64 (7.2) | |||
| Previous | 6 (1.9) | 4 (2.5) | 2 (1.3) | 365 (41.1) | |||
| Hypertension | 185 (58.2) | 89 (55.6) | 96 (60.8) | 526 (59.2) | 0.35 | 0.61 | 0.74 |
| Dyslipidemia | 141 (44.3) | 65 (40.6) | 76 (48.1) | 603 (67.9) | 0.18 | <0.001 | <0.001 |
| Family history of CAD | 82 (25.8) | 44 (27.5) | 38 (24.1) | 305 (34.3) | 0.48 | 0.02 | 0.005 |
| Diabetes mellitus | 37 (11.6) | 11 (6.9) | 26 (16.5) | 229 (25.8) | 0.008 | <0.001 | <0.001 |
| Previous MI | 19 (6.0) | 10 (6.3) | 9 (5.7) | 201 (22.6) | 0.84 | <0.001 | <0.001 |
| Previous stroke | 8 (2.5) | 2 (1.3) | 6 (3.8) | 44 (5.0) | 0.17 | 0.10 | 0.08 |
| Previous PCI | 15 (4.7) | 7 (4.4) | 8 (5.1) | 187 (21.1) | 0.77 | <0.001 | <0.001 |
| Previous CABG | 3 (0.9) | 1 (0.6) | 2 (1.3) | 71 (8.0) | 0.62 | <0.001 | <0.001 |
| Atrial fibrillation | 31 (9.7) | 16 (10.0) | 15 (9.5) | 31 (3.5) | 0.88 | <0.001 | <0.001 |
| Previous peptic ulcer | 21 (6.6) | 12 (7.5) | 9 (5.7) | 119 (13.4) | 0.52 | 0.005 | 0.001 |
| LVEF | 54 ± 9 | 55 ± 11 | 54 ± 9 | 51 ± 9 | 0.53 | <0.001 | <0.001 |
| Chronic renal failure | 41 (12.9) | 13 (8.1) | 28 (17.7) | 89 (10.0) | 0.01 | 0.008 | 0.16 |
| Admission diagnosis | <0.001 | <0.001 | <0.001 | ||||
| STEMI | 56 (17.6) | 39 (24.4) | 17 (10.8) | 372 (41.9) | |||
| NSTEMI | 207 (65.1) | 84 (52.5) | 123 (77.8) | 295 (33.2) | |||
| Unstable angina | 55 (17.3) | 37 (23.1) | 18 (11.4) | 221 (24.9) | |||
| Glycoprotein IIb/IIIa inhibitors | 91 (28.6) | 29 (18.1) | 62 (39.2) | 423 (47.6) | <0.001 | <0.001 | <0.001 |
| Radial access | 138 (43.4) | 87 (54.4) | 51 (32.3) | 121 (13.6) | <0.001 | <0.001 | <0.001 |
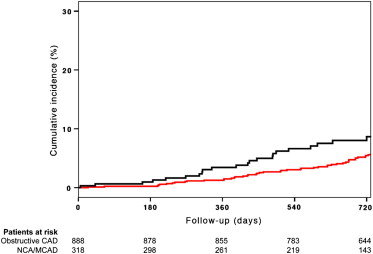
The incidence of MACE at 30 days was low in all groups and was similar between the NCA/MCAD and obstructive CAD groups (0.3% vs 0.1%, p = 0.46; Table 2 ). In contrast, the patients with obstructive CAD experienced greater rates of MACE at 26 ± 16 months of follow-up (16.6% vs 9.1% p = 0.001; Figure 1 ), which was driven by a greater rate of myocardial infarction than those without (5.3% vs 0%, p <0.001; Table 2 ). No differences were found in the rate of death, ACS leading to hospitalization, stroke, and bleeding (both Thrombolysis In Myocardial Infarction major and minor) between the 2 groups ( Table 2 ). Among the patients with NCA/MCAD, no difference was found in the incidence of MACE between those with NCA and MCAD both at 30 days (p = 0.50) and at long-term follow-up (p = 0.16). However, the patients with NCA were less likely to have ACS leading to hospitalization than those with MCAD (1.2% vs 6.3%, p = 0.02). After adjusting for baseline characteristics, no difference was found in the risk of MACE at follow-up across the 3 groups ( Table 3 ). The adjusted risk of MACE at follow-up was similar in the male and female patients ( Table 4 ), with no significant gender by group interaction (p for interaction = 0.37).
| Outcome | NCA/MCAD (A+B; n = 318) | NCA (A; n = 160) | MCAD (B; n = 158) | Obstructive CAD (C; n = 888) | p Value | |
|---|---|---|---|---|---|---|
| A vs B | A+B vs C | |||||
| 30-Day | ||||||
| MACE | 1 (0.3) | 0 (0) | 1 (0.6) | 1 (0.1) | 0.50 | 0.46 |
| Major bleeding | 6 (1.9) | 2 (1.3) | 4 (2.5) | 14 (1.6) | 0.45 | 0.71 |
| Minor bleeding | 10 (3.1) | 7 (4.4) | 3 (1.9) | 39 (4.4) | 0.34 | 0.33 |
| Follow-up (26 ± 16 mo) | ||||||
| MACE | 29 (9.1) | 11 (6.9) | 18 (11.4) | 147 (16.6) | 0.16 | 0.001 |
| Death | 14 (4.4) | 7 (4.4) | 7 (4.4) | 54 (6.1) | 0.98 | 0.27 |
| MI | 0 (0) | 0 (0) | 0 (0) | 47 (5.3) | – | <0.001 |
| Destabilizing cardiac symptoms leading to hospitalization | 12 (3.8) | 2 (1.2) | 10 (6.3) | 41 (4.6) | 0.02 | 0.53 |
| Stroke | 3 (0.9) | 2 (1.2) | 1 (0.6) | 5 (0.6) | 1.00 | 0.44 |
| Major bleeding | 11 (3.5) | 6 (3.8) | 5 (3.2) | 40 (4.5) | 0.78 | 0.43 |
| Minor bleeding | 21 (6.6) | 13 (8.1) | 8 (5.1) | 69 (7.8) | 0.27 | 0.50 |
| OR | 95% CI | p Value | |
|---|---|---|---|
| Unadjusted | |||
| NCA ∗ | – | – | – |
| MCAD | 1.11 | 0.65–1.90 | 0.70 |
| Obstructive CAD | 0.79 | 0.43–1.44 | 0.44 |
| Adjusted | |||
| NCA ∗ | – | – | – |
| MCAD | 0.79 | 0.33–1.87 | 0.59 |
| Obstructive CAD | 0.62 | 0.30–1.29 | 0.20 |
| OR | 95% CI | p Value | |
|---|---|---|---|
| Male gender | |||
| NCA ∗ | – | – | – |
| MCAD | 0.41 | 0.05–3.32 | 0.41 |
| Obstructive CAD | 0.62 | 0.20–1.86 | 0.39 |
| Female gender | |||
| NCA ∗ | – | – | – |
| MCAD | 1.31 | 0.46–3.78 | 0.61 |
| Obstructive CAD | 0.80 | 0.29–2.22 | 0.67 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


