Severely symptomatic patients with obstructive hypertrophic cardiomyopathy (HC) may benefit from surgical myectomy. In patients with enlarged mitral leaflets and mitral regurgitation, myectomy can be combined with anterior mitral leaflet extension (AMLE) to stiffen the midsegment of the leaflet. The aim of this study was to evaluate the long-term results of myectomy combined with AMLE in patients with obstructive HC. This prospective, observational, single-center cohort study included 98 patients (49 ± 14 years, 37% female) who underwent myectomy combined with AMLE from 1991 to 2012. End points included all-cause mortality and change in clinical and echocardiographic characteristics. Mortality was compared with age- and gender-matched patients with nonobstructive HC and subjects from the general population. Long-term follow-up was 8.3 ± 6.1 years. There was no operative mortality, and New York Heart Association class was reduced from 2.8 ± 0.5 to 1.3 ± 0.5 (p <0.001), left ventricular outflow tract gradient from 93 ± 25 to 9 ± 8 mm Hg (p <0.001), mitral valve regurgitation from grade 2.0 ± 0.9 to 0.5 ± 0.8 (p <0.001), and systolic anterior motion of the mitral valve from grade 2.4 ± 0.9 to 0.1 ± 0.3 (p <0.001). The 1-, 5-, 10-, and 15-year cumulative survival rates were 98%, 92%, 86%, and 83%, respectively, and did not differ from the general population (99%, 97%, 92%, and 85%, respectively, p = 0.3) or patients with nonobstructive HC (98%, 97%, 88%, and 83%, respectively, p = 0.8). In conclusion, in selected patients with obstructive HC, myectomy combined with AMLE is a low-risk surgical procedure. It results in long-term symptom relief and survival similar to the general population.
The aim of this study was to evaluate the long-term results of myectomy combined with anterior mitral leaflet extension (AMLE) in patients with obstructive hypertrophic cardiomyopathy (HC). In the present report, long-term outcome after myectomy combined with AMLE was compared with age- and gender-matched patients with nonobstructive HC and subjects from the general Dutch population.
Methods
The study conforms to the principles of the Declaration of Helsinki. All patients gave informed consent for the intervention and prospective inclusion in a registry, and local institutional review board approval was obtained. A total of 139 patients with obstructive HC underwent surgical myectomy from 1991 to December 2012. Myectomy with AMLE was performed in 98 patients (71%), isolated myectomy in 24 patients (17%), and myectomy combined with mitral valve replacement (MVR) in 14 patients (12%).
Patients are selected for surgery at our HC center on the basis of the following indications: (1) peak left ventricular outflow tract (LVOT) gradient ≥50 mm Hg at rest or on provocation and (2) presence of unacceptable symptoms despite maximally tolerated medications consisting of β-blocking agents and/or calcium channel blockers. The decision to perform surgery was made after consensus of a heart team consisting of a cardiothoracic surgeon, an interventional cardiologist, and a cardiologist specialized in HC care.
Myectomy combined with AMLE is performed in patients with enlargement of the anterior mitral valve area (>12 cm 2 ), calculated with the formula previously validated by Klues et al. The surgical technique has been described previously. In brief, an autologous pericardial patch is harvested, trimmed of fat and extraneous tissue, immersed for 6 minutes in 0.4% glutaraldehyde, and then placed in a normal saline bath. After opening the ascending aorta by an oblique incision, myectomy is performed to the left of an imaginary line through the nadir of the right coronary cusp in the beginning with a locally designed electrocautery device, later by excision with scissors and a rongeur or surgical knife. After myectomy, AMLE is performed. A gap is created in the anterior mitral leaflet by a longitudinal incision, starting at the subaortic hinge point and ending just before the rough zone. Then, an oval autologous pericardial patch, of about 2.5 cm wide and 3 cm long, is grafted across the bending point of the mitral valve where the systolic anterior motion (SAM) is maximal to stiffen the buckling anterior mitral valve leaflet (AMVL). The patch extends the width but not the length of the AMVL, which shifts the centrally attached chordae laterally. As a result, the chordae are stretched and erected, which will enhance leaflet coaptation. Finally, because force produced by blood flow against the leaflet is proportional to its area, the increased leaflet will be pressed posterior, with a decrease in SAM and mitral valve regurgitation (MR). The surgical results were assessed with transesophageal echocardiography immediately after weaning from cardiopulmonary bypass and at a systolic blood pressure of ≥100 mm Hg.
The clinical characteristics collected before the intervention included assessment of symptoms, New York Heart Association (NYHA) functional class, and prescribed drugs. Physical examination and baseline laboratory studies were performed, including electrocardiography, transthoracic echocardiography, and cardiac catheterization. The echocardiographic data were reviewed by a physician unaware of the patient’s medical history. Echocardiography was performed in the month before surgery and after surgery repeated at 1 week, 3 months, and at yearly intervals. Ventricular septum thickness was calculated at the site of myectomy from the septal width in diastole from both the parasternal short- and long-axis views. The severity of the MR was graded on a 0 to 4 scale by color flow Doppler echocardiography. The severity of the SAM of the AMVL was determined from the 2-dimensional images and was graded on a scale from 0 to 3 depending on the mitral-septal distance (grade 0 indicating no SAM and grade 3 indicating prolonged contact between mitral valve and septum). The length of the AMVL was noted. Peak LVOT gradient was estimated with Doppler echocardiography by the modified Bernoulli equation (P = 4v 2 ), where P is the pressure gradient and v is Doppler-determined blood velocity.
The primary end point was all-cause mortality. Follow-up information was obtained at routine visits at the HC outpatient clinic. For 7 patients, follow-up information was collected by their referring cardiologist. Follow-up vital status and cause of death were obtained by reviewing the hospital records, from general practitioners and civil registries. Sudden cardiac death (SCD) was defined as instantaneous and unexpected death within 1 hour after a witnessed collapse in patients who previously were in stable clinical condition, or nocturnal death with no antecedent history of worsening symptoms. Follow-up data were complete for all patients.
All statistics were performed using the SPSS 21 (IBM, Armonk, New York) and Microsoft Excel 2010 (Microsoft Corporation, Redmond, Washington). Data are expressed as mean ± SD or number (percentage). The normal distribution for continuous data was examined with the Shapiro-Wilk test. Comparison of numerical variables was performed using the 2-sided Student t test, Wilcoxon rank-sum test, or analysis of variance, and the chi-square or Fisher’s exact tests were used to compare qualitative variables. The p values are 2-sided; p <0.05 was considered statistically significant. The survival analysis model used proportional hazards regression methodology; Kaplan-Meier survival curves were compared using log-rank statistics. Long-term survival of patients who underwent myectomy combined with AMLE was compared with age- and gender-matched patients with nonobstructive HC and with the expected survival curve for the general Dutch population. This expected survival curve was generated from the database of Statistics Netherlands, which incorporates all-cause mortality ( www.cbs.nl ). The administrative censoring date was set at August 1, 2013. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the report as written.
Results
A total of 343 patients with obstructive HC (LVOT gradient >50 mm Hg) were evaluated at our center. Of these patients, 139 (41%) underwent myectomy, 97 (28%) underwent alcohol septal ablation (ASA), and 107 (31%) were treated medically. The majority of medically treated patients (n = 91, 85%) reported no symptoms or mild (NYHA class I/II) symptoms at baseline, despite a mean LVOT gradient of 70 ± 24 mm Hg. The other 16 patients (15%) had an indication for invasive treatment but were considered not eligible because of severe co-morbidities (e.g., 1 patient had liver cirrhosis due to alcohol abuse and kidney failure) or patient refusal (several patients refused further invasive treatment, mostly because they were at old age and preferred no further interventions).
The baseline characteristics of the 98 combined surgery patients and 24 isolated myectomy patients are listed in Table 1 . Myectomy with additional coronary bypass grafting was performed in 3 patients (3%). In 14 patients, myectomy and MVR was performed directly, instead of AMLE. Reasons for direct MVR were (among others): chordal rupture or prolapse of posterior leaflet, severe calcification of the valvular apparatus, and infective endocarditis with valvular destruction. In most of these cases, MVR was planned before surgery, but the final decision to perform AMLE was made intraoperatively by the surgeon after epicardial and/or transesophageal beating heart echocardiography and visual inspection of the arrested heart. In 8 patients (8%) myectomy and AMLE was performed after failed ASA or embolization using coils.
| Variable | Combined myectomy (n=98) | Isolated Myectomy (n=24) | Non-obstructive (n=98) |
|---|---|---|---|
| Age (years) | 49 ± 14 | 48 ± 18 | 49 ± 15 |
| Female | 36 (37%) | 10 (42%) | 36 (37%) |
| NYHA III/IV | 73 (74%) | 19 (79%) | 10 (10%) ∗∗∗ |
| LV wall thickness (mm) | 22 ± 5 | 20 ± 3 | 20 ± 5 ∗ |
| LV outflow tract gradient (mmHg) | 93 ± 25 | 83 ± 20 | 8 ± 5 ∗∗∗ |
| Anterior mitral leaflet length (mm) | 34 ± 4 | 29 ± 3 ∗∗∗ | 28 ± 4 ∗∗∗ |
| Mitral regurgitation | 2.0 ± 0.9 | 1.3 ± 0.9 ∗∗∗ | 0.5 ± 0.7 ∗∗∗ |
| Medication | |||
| β-receptor antagonist | 67 (68%) | 19 (79%) | 38 (39%) ∗∗∗ |
| Calcium-channel blocker | 48 (49%) | 13 (54%) | 12 (12%) ∗∗∗ |
| Follow-up | |||
| Duration, (years) | 8.3 ± 6.1 | 5.0 ± 6.0 ∗ | 10.6 ± 5.5 ∗ |
| Cause of death | |||
| – Heart failure | 6 (6%) | 0 | 4 (4%) |
| – Sudden death | 3 (3%) | 0 | 3 (3%) |
| – Non-cardiac | 1 (1%) | 1 (4%) | 6 (6%) |
Because advanced symptoms refractory to pharmacologic therapy represent the standard indication for operation, patients who underwent myectomy (both isolated and combined with AMLE) expectedly showed more severe functional disability at study entry than patients with nonobstructive HC (74% to 79% in NYHA class III or IV compared with 7% in the nonobstructive group; p <0.001). The length of the AMVL before surgery was longer in patients who underwent combined surgery, compared with the isolated myectomy and nonobstructive patients. MR (before surgery) was also more severe in patients who underwent combined surgery ( Table 1 ).
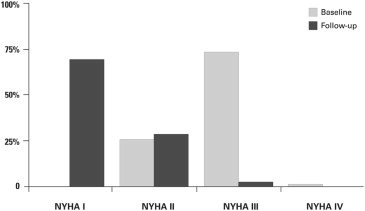
The myectomy combined with the AMLE group experienced substantial symptomatic and hemodynamic improvement after surgery; 73 patients (74%) were in NYHA functional class III or IV before operation, whereas only 2 of 89 (2%) remaining patients at latest follow-up were in NYHA class III ( Figure 1 ). Compared with preoperative data, there were significant changes in peak LVOT gradient, MR severity, and presence of SAM of the mitral valve at latest follow-up compared with preoperative state ( Table 2 ).
| Variable | Baseline (n=98) | Follow-up (n=86) | P |
|---|---|---|---|
| Ventricular septum (mm) | 22 ± 5 | 16 ± 4 | < 0.001 |
| LV end diastolic diameter (mm) | 44 ± 6 | 49 ± 7 | < 0.001 |
| LV outflow tract gradient (mmHg) | 93 ± 25 | 9 ± 8 | < 0.001 |
| Left atrial size (mm) | 48 ± 8 | 49 ± 11 | 0.5 |
| Systolic anterior motion (grade) | 2.4 ± 0.9 | 0.1 ± 0.3 | < 0.001 |
| Mitral valve regurgitation (grade) | 2.0 ± 0.9 | 0.5 ± 0.8 | < 0.001 |
Long-term follow-up was 8.3 ± 6.1 years or 809 patient-years. Four patients (2 of those were operated after failed ASA) developed complete heart block during surgery. Three patients received a permanent pacemaker, in the remaining patient combined with an implantable cardioverter-defibrillator (ICD) because of a high risk status for SCD. An ICD was implanted in 17 more patients for primary prevention of SCD. One patient received appropriate ICD shocks during follow-up. Paroxysmal or persistent atrial fibrillation (AF) occurred in 36 patients (37%) during follow-up. Of these patients, in 21 (21%), AF occurred during follow-up, and 15 patients (15%) only had an episode of postoperative AF (6 of these had a history of AF). Electric cardioversion during postoperative recovery was necessary in 7 patients (7%).
None of the patients had an indication for reinstitution of cardiopulmonary bypass. Perfect mitral competence (grade 0 MR) was present in 57 patients (58%), and only 2 (2%) had grade 3+ MR at latest follow-up. In 1 patient (1%), early valve repair failure, due to rupture of the new chordae (13 days after initial surgery), leading to MVR was necessary. Other indications of resurgery are listed in Table 3 .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


