Chapter 46
Local Complications
Endovascular
Elliot B. Sambol, James F. Mckinsey
There is continued growth in adaptation as well as complexity of endovascular techniques for the management of arterial and venous disease by today’s vascular interventionists. The endovascular intervention, despite its clearly proven advantages, also has a new class of complications. Although many of these complications are minor and can be treated with endovascular techniques, there is also the potential for serious life- or limb-threatening conditions requiring urgent complex treatment with either open surgical or percutaneous techniques. The purpose of this chapter is to describe the more commonly encountered complications of endovascular procedures, including strategies for prevention and their management.
Femoral Access Site Complications
More than 95% of all percutaneous peripheral interventions are performed by access via the femoral arteries.1 There is an increasing use of radial artery access for percutaneous coronary intervention (PCI), and this may become the dominant access technique in the future. Problems at these access sites are the most common adverse events encountered in peripheral and coronary interventions and can lead to significant morbidity and even mortality. Because of the large volume of PCI procedures performed in the United States, data on access site complications are derived mostly from this patient population. Nevertheless, access at the groin is similar whether it is performed for PCI or peripheral interventions, and therefore considerable information can be extrapolated from these studies. The most recent PCI data reveal an overall incidence of access site injury ranging between 2.1% and 6.6% when it is defined as a complication requiring procedural or surgical intervention or as bleeding requiring transfusion.2–5 In 2012, Smilowitz et al6 retrospectively reviewed 9108 transfemoral PCI procedures comparing manual compression (2581; 28.3%) with vascular closure devices (6527; 71.7%). They reported 74 (0.81%) significant complications, with 32 (1.24%) complications in patients treated with manual compression and 42 (0.64%) in patients treated with closure devices. In addition, they reported a 1.23% closure device failure. Closure device use was a predictor of fewer complications (odds ratio, 0.52; 95% confidence interval, 0.33 to 0.83). Piper et al7 analyzed the northern New England PCI registry and found a 3% rate of vascular complications in 18,137 consecutive patients. Table 46-1 lists access site complications in order of decreasing frequency.
Table 46-1
Complications of Percutaneous Access
| Complication | Incidence (%) |
| Bleeding or hematoma8 | 1.2-8.9 |
| Pseudoaneurysm9,10 | 1.1-7.7 |
| Arteriovenous fistula11 | 0.86 |
| Dissection13 | 0.42 |
| Thrombosis8,12 | 0.07-0.1 |
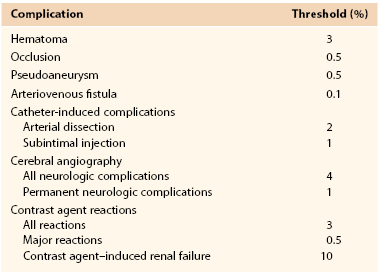
Predicting which patients are at greatest risk for access site injury is crucial for the endovascular surgeon. The most notable risk factors for access site complications include larger sheath size, interventional procedures, arteriotomy site, arterial characteristics, previous catheterization, smaller body mass index, female gender, uncontrolled hypertension, over-anticoagulation, glycoprotein IIb/IIIa inhibitor use, and increased age.2,4,7,14–17 By identifying groups of higher risk patients, risk reduction strategies can be implemented and complications can be decreased. Such strategies include weight-based procedural anticoagulation, tight blood pressure control, and strict adherence to proper percutaneous technique (discussed in Chapters 88 and 89). Fitts et al18 reported an almost threefold reduction in pseudoaneurysm formation and a significantly lower risk for all arterial injuries (1.9% vs 0.7%; P < .01) by simply identifying the femoral head fluoroscopically before puncture. Despite even the best techniques and lowest risk patients, however, percutaneous interventions can be fraught with problems. Therefore, the Society for Interventional Radiology has set benchmarks by reporting acceptable threshold incidences for these complications (Table 46-2).
Groin Hematoma
A frequent complication of percutaneous access is bleeding and the development of a hematoma. This multifactorial problem is influenced by many periprocedural factors, and early recognition and proper treatment can mitigate significant morbidity. Significant risk factors for the development of a groin hematoma include female gender, age older than 65 years, and the use of aspirin, clopidogrel, and glycoprotein IIb/IIIa inhibitors, which have been shown to present a modest increased risk for bleeding complications.7,17,19 However, the medical benefit conferred by these antiplatelet agents outweighs the bleeding risk in such patients undergoing PCI. In most cases, patients requiring percutaneous peripheral or coronary interventions should not be instructed to stop their antiplatelet regimens. For interventionists, these patients should be classified as being at higher risk for bleeding, and therefore higher vigilance for groin complications should be implemented.
Etiology and Manifestations
Unsuccessful femoral artery access attempts can result in periarterial hematoma that obscures the palpation of the femoral pulse, making percutaneous access even more difficult. It is a common practice to maintain at least 5 minutes of manual pressure if the artery has been punctured without successful access before a repeated attempt. Back wall injury by the needle can result in extravasation of blood that continues throughout the procedure, especially in the presence of anticoagulation. Accumulation of blood can also occur during the procedure while the sheath is intraluminal because of leakage around the catheter. This complication may result from access through a calcified anterior arterial wall and resultant creation of an asymmetric or stellate arteriotomy. The circular sheath is not hemostatic in these circumstances, and manipulation of the sheath in conjunction with wire, catheter, and larger sheath exchanges can exacerbate the hematoma expansion. Early recognition of this complication allows appropriate upsizing of the sheath and prevention of large hematomas.
The clinical manifestation of a groin hematoma varies widely. In most circumstances, postprocedure groin swelling is the only clinical finding. Other common signs and symptoms include pain, skin ecchymosis, bleeding at the puncture site, neuropathy secondary to femoral nerve compression, anemia, hypotension, and, in the most severe cases, shock.
Management
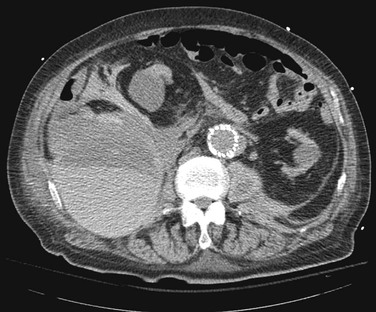
Treatment strategies for groin hematomas generally consist of observation along with correction of any underlying coagulopathy, stoppage of anticoagulation, and transfusion to maintain appropriate hemoglobin levels. Manual compression should be applied to hematomas that have been identified in the acute setting, with specific attention to focused digital pressure directly at the site of puncture. The placement of “sandbag” type pressure on the groin is generally ineffective because it will not place direct pressure on the bleeding femoral vessel but rather displace the force throughout the groin. Mechanical compression devices, such as the FemoStop and C-clamp, have demonstrated benefit in reducing bleeding complications.20 The extremity in which the mechanical compression device is applied should be carefully monitored to ensure that it does not slip and become displaced off the femoral vessels or compress the femoral artery to the point of occlusion and thrombosis. Computed tomography (CT) is useful to gauge the extent and expansion of the hematoma and to assess for active bleeding as evidenced by a contrast-induced blush (Fig. 46-1). Patients should be placed on bed rest and undergo frequent physical examination to ensure that the size of the hematoma is stable. Continued hemodynamic instability without groin hematoma expansion could indicate expansion of the hematoma into the retroperitoneal space, and further CT scan or duplex ultrasound evaluation is indicated. Laboratory assessment of hemoglobin levels should be done every 4 to 6 hours until stabilization. Once the hematoma is stable, a duplex ultrasound assessment should be performed to rule out the development of a pseudoaneurysm.
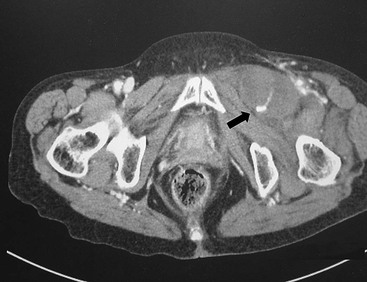
Figure 46-1 CT scan demonstrating a postangiography left groin hematoma with a contrast blush (arrow) suggesting active bleeding. The patient required open exploration and repair because of femoral nerve compression.
Although most of these patients can avoid surgical exploration, the morbidity from a hematoma is significant and includes skin changes, ecchymosis, prolonged pain, and retroperitoneal bleeding. In addition, the time to ambulation is delayed and length of stay increases. Furthermore, patients receiving transfusion for groin hematoma have been shown to have significantly higher mortality rates than those who do not require transfusion.21
Groin exploration with evacuation of the hematoma is warranted in the setting of hemodynamic instability, persistent anemia despite ongoing transfusions, skin necrosis, nerve compression, or severe pain. Surgery is often challenging because tissue planes have been destroyed by a dissecting hematoma, and therefore postoperative care is fraught with wound complications. The femoral artery should be explored thoroughly and the arteriotomy inspected for active bleeding, including examination of the posterior wall of the common femoral artery (CFA) to ensure that no back wall injury has occurred.
Retroperitoneal and Inguinal Canal Hematoma
High punctures at the groin can result in an increased risk for the development of access site complications, including retroperitoneal hematoma16 and inguinal canal hematoma. Although it is rare, retroperitoneal hematoma has the potential for severe morbidity and even death if it is not detected. Bleeding that extends into the retroperitoneum is less likely to be tamponaded by surrounding structures. The retroperitoneal space can accommodate a large quantity of blood, which can ultimately compress adjacent organs, nerves, and soft tissue (Fig. 46-2). If the arterial puncture is close to or involves the inguinal ligament, the hematoma can track along the spermatic cord into the scrotum, resulting in scrotal hematoma. In rare cases, impingement on the testicular vessels and testicular infarction may ensue.
Manifestations and Diagnosis
Clinical findings are often subtle and less obvious than with groin hematomas. Patient complaints of nonspecific groin, back, or lower abdominal pain should raise suspicion for retroperitoneal hematoma after a percutaneous procedure. A falling hematocrit, hypotension, and oliguria—all signs of active bleeding—should prompt rapid imaging to rule out retroperitoneal hematoma. Other symptoms of retroperitoneal hematoma include thigh pain, numbness or weakness that could be secondary to femoral nerve compression in the pelvis, lower abdominal quadrant fullness, and ecchymosis at the flanks (Grey Turner sign) or the umbilicus (Cullen’s sign).
The diagnostic test of choice for retroperitoneal hematoma is abdominopelvic CT (Fig. 46-3). If it is performed with the intravenous administration of contrast material, extravasation can be an indicator of active bleeding and may prompt earlier intervention. Retroperitoneal hematoma can often be distinguished from spontaneous retroperitoneal bleeding by communication of the retroperitoneal hematoma with the femoral vessels. Spontaneous retroperitoneal hematoma bleeding is often more centrally located and generally venous in etiology. Wire injuries to the vena cava or iliac veins can also result in retroperitoneal hematoma, but surgical intervention is required only for ongoing blood loss or abdominal compartment syndrome.
Management
Therapy for retroperitoneal hematoma is tailored to the clinical status of the patient. In most cases, retroperitoneal hematomas can be managed conservatively with serial hematocrit determinations, correction of coagulopathy, and transfusions as needed. Similar to patients with groin hematomas, patients should be placed on bed rest in a monitored setting. Indications for intervention include neurologic deficits in the affected extremity, hemodynamic instability, ongoing blood loss, and severe pain.22 When it is necessary, decompression of a retroperitoneal hematoma can be performed either through a groin incision or through an incision above the inguinal ligament to gain direct access to the retroperitoneum and iliac vessels. Either way, the arteriotomy must be investigated and repaired for optimal therapy.
Symptomatic inguinal canal hematoma originating from a percutaneous CFA access and pseudoaneurysm formation can be treated by duplex ultrasound–directed compression, surgical exploration, or duplex ultrasound–directed thrombin injection into the pseudoaneurysm sac.
Arteriovenous Fistula
Etiology, Manifestations, and Incidence
An arteriovenous fistula (AVF) in the groin is a rare complication of percutaneous access and involves a communication between the femoral artery and vein. It can occur when a simultaneous right- and left-sided heart catheterization is performed to access the femoral artery and vein. It can also occur after inadvertent low puncture of the CFA bifurcation or between the profunda femoris artery and profunda femoral vein, which run near the superficial femoral artery (SFA). AVF may also develop after through-and-through puncture of the CFA or SFA into the common femoral vein. Groin AVFs are generally asymptomatic and detected on physical examination by the presence of a palpable thrill in the groin or auscultation of a continuous bruit. Rarely do AVFs result in cardiac failure because the shunt volume from these fistulae (average of 160 to 510 mL/min) falls far below that required to cause significant cardiac dysfunction.11 Duplex ultrasound is the imaging study of choice and demonstrates the characteristic systolic-diastolic flow pattern with arterialization of the venous signal (Fig. 46-4).23
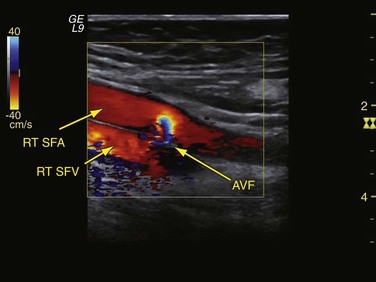
Figure 46-4 Duplex image of an iatrogenic arteriovenous fistula (AVF) caused by inadvertent through-and-through puncture of the right superficial femoral artery (RT SFA) into the right superficial femoral vein (RT SFV).
The incidence of AVF after catheterization varies by study and is significantly influenced by the evaluation criteria as well as by the study cohort, inasmuch as many of these patients had been referred to vascular surgery for surgical repair. Kresowik et al24 prospectively performed primary duplex scans on 144 patients undergoing PCI and found four AVFs (2.8%); all were asymptomatic. In contrast, Ricci et al25 identified only four AVFs, all symptomatic, in 7690 catheterizations (0.05%). In one of the larger studies to date, Kelm et al11 evaluated 10,271 consecutive patients undergoing PCI with a 3-year follow-up and reported a 0.88% incidence of AVF. In accordance with the work of Kent et al,26 who described a 0.3% incidence of AVF, these investigators used an audible bruit in the groin as the trigger to prompt duplex ultrasound investigation. Risk factors for the development of AVF included female gender and hypertension, and procedure-related factors included puncture of the left groin and high-dose procedural anticoagulation.27 The reason for the elevated risk for AVF with left groin puncture is believed to be the angle of approach because the physician generally stands on the contralateral side of the patient.23
Management
Because of the rarity of this phenomenon, the natural history of femoral AVFs is somewhat unclear, and therefore the treatment algorithm is controversial. Treatment options include observation alone, surgical repair, and endovascular repair. Ultrasound-guided compression has been reported to treat groin AVF; however, low success rates have prompted abandonment of this therapy.28 Kelm et al11 showed that 38% of AVF patients underwent spontaneous closure of their fistula within 1 year and that more than two thirds of these fistulae closed within 4 months. Of the remaining fistulae that persisted, the authors reported no adverse outcomes, including right-sided heart failure or limb swelling. Similarly, Toursarkissian et al29 monitored 81 AVFs, 81% of which closed spontaneously, prompting the authors to recommend that carefully selected patients can be managed without surgery or intervention. If conservative management is implemented, patients require close duplex ultrasound surveillance and frequent physical examination evaluating for signs of congestive heart failure or limb swelling. If symptoms do arise or the fistula increases in size, surgical treatment is indicated.
Groin exploration can in most cases be performed with local anesthesia. AVF ligation consists of standard proximal and distal control of the artery, followed by division of the fistula and primary closure of the defect in both vessels with interrupted polypropylene sutures. Depending on chronicity, there is always the potential for large-volume scar tissue to have developed, which can at times make this procedure more complex.
Endovascular management of femoral AVFs has been reported with balloon-expandable stent-grafts.30–32 Coverage of the arterial component of the fistula with a covered stent-graft results in immediate abolishment of the AVF without the need for surgery and therefore is optimal for high-risk candidates who may have failed to respond to conservative management. Onal et al33 were clinically successful in abolishing 9 of 10 AVFs originating from the profunda femoris artery, and all stents were patent at 18 months of follow-up. However, caution must be exercised in deployment of these stents, especially in the distal CFA or the proximal profunda femoris, where the graft could impinge on the origin of the SFA or profunda femoris artery. In addition, the long-term durability of covered stents in this anatomic location remains to be determined. Another endovascular option is embolization of long fistula tracks with n-butyl cyanoacrylate, but there is a risk of distal embolization of the agent.34 The decision whether to treat an AVF with open, endovascular, or conservative measures is still under debate and should be driven by the surgeon’s experience and the patient’s overall symptoms and comorbid conditions.
Pseudoaneurysm
Etiology and Manifestations
A pseudoaneurysm (PSA) develops after endovascular procedures if the arteriotomy has not adequately sealed once the sheath is removed or if the occluding clot in the arteriotomy is dislodged in the postprocedure period. There is communication with the arterial lumen, and blood extravasates into the surrounding soft tissue. It is a “pseudo” aneurysm in that no elements of the arterial wall are incorporated in the aneurysm sac; the wall of the PSA consists solely of compressed thrombus and surrounding soft tissue. PSAs can be differentiated from hematomas in that there is arterial flow into the PSA with a defined neck that tracks to the arteriotomy through the femoral sheath. The incidence of PSA formation after percutaneous procedures varies widely from 0.05% to 8.0%, with heterogeneity of study characteristics being the main cause of this variability.24,35,36 Factors that increase the risk for PSA are listed in Box 46-1 and are similar to those for other postcatheterization vascular complications.
The cause of a PSA is often related to an inability to adequately compress the vessel after removal of the sheath. This occurs most frequently with SFA or low CFA puncture because the femoral head sits more cephalic and compression is inadequate. To decrease the potential for PSA, it is important to identify the femoral head fluoroscopically before puncture so that the arteriotomy site will be placed over the bone. In a large meta-analysis by Koreny et al37 involving more than 4000 patients, use of a vascular closure device was also associated with a slightly higher risk for PSA formation than was manual compression alone.
PSAs are characterized by a pulsatile mass at the puncture site, usually within 24 to 48 hours after the procedure. They are often associated with groin tenderness and can be difficult to differentiate from hematomas. Large PSAs can cause local compression symptoms on the femoral nerve, leading to a neuropathy, or on the femoral vein, leading to thrombosis. Large PSAs can also cause local skin ischemia with eventual necrosis. On physical examination, a systolic bruit can be auscultated and is associated with the pulsatile groin mass.
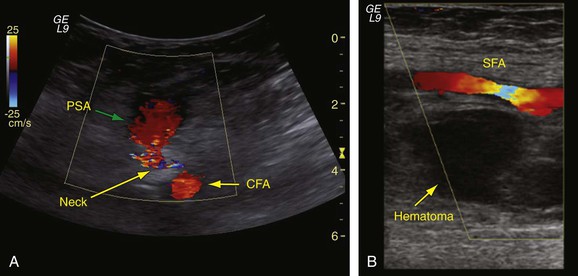
When there is clinical suspicion of a PSA, groin arterial duplex ultrasound examination should be performed. Duplex ultrasound examination provides a determination of the size and location of the PSA relative to the bifurcation of the CFA to the profunda and SFA and also allows differentiation between hematoma and PSA, the latter having arterial flow characteristics outside the vessel. The typical ultrasound appearance of a PSA is an echolucent sac that is pulsatile, and with the addition of color Doppler study, a swirling flow pattern is noted within this sac (Fig. 46-5). On spectral waveform analysis, the characteristic “to-and-fro” flow pattern can be discerned and is pathognomonic for PSA.38 Adjacent structures can also be visualized with B-mode imaging, and after the artery is localized, the neck of the aneurysm can be found as it communicates with the sac. The sensitivity of duplex ultrasound for the identification of PSA is 94% with a specificity of 97%.39 If the PSA has significant extension into the retroperitoneum, abdominopelvic contrast-enhanced CT may be of benefit to evaluate its size as well as to identify additional arterial injury sites.
Management
Ultrasound-Guided Compression.
Management of iatrogenic PSAs has changed during the last 2 decades. Before the 1990s, surgery was the primary recommended therapy for large or expanding PSAs to decrease the complications of nerve compression, distal embolization, and skin necrosis. Since that time, however, multiple less invasive treatment strategies have been proposed. In 1991, Fellmeth et al40 described the technique of ultrasound-guided compression of femoral PSAs, which resulted in the obliteration of 27 of 29 lesions. The technique involves placement of an ultrasound probe directly over the neck of the PSA and application of downward pressure until flow is abolished in the aneurysm sac. It is important not to compress so aggressively that flow through the native vessel is interrupted and thrombosis could ensue. After 10 minutes of pressure, the sac is assessed with color duplex ultrasound, and if flow is still present, pressure is maintained for another 20 minutes. These cycles are repeated at the discretion of the operator. In the largest series published to date, success rates ranged from 63% to 88%.41–43 The main factors impeding successful ultrasound-guided compression include anticoagulation at the time of compression, size of the PSA, and patient discomfort. Risks associated with ultrasound-guided compression include PSA rupture, distal embolization, and thrombosis of the femoral vein or artery.43
Ultrasound-Guided Thrombin Injection.
The disadvantages of ultrasound-guided compression, including patient and ultrasound technician discomfort and only moderate success, prompted the development of a new therapy consisting of injection of thrombin directly into the PSA under ultrasound guidance. Cope and Zeit44 in 1986 reported successful treatment of PSAs arising from the common iliac artery, the peroneal artery, the hepatic artery, and the proximal anastomosis of a femoropopliteal graft. The principle of this technique is based on the necessity of thrombin for conversion of fibrinogen to fibrin. When blood is exposed to high-dose thrombin within the aneurysm sac, fibrin clot forms and instantaneously results in thrombosis of the PSA. Numerous studies have evaluated the efficacy of ultrasound-guided thrombin injection for PSA thrombosis (Table 46-3),45–59 and on the basis of these very convincing data, this modality has now become a first-line treatment strategy for PSAs that do not otherwise require emergency surgical repair or can be managed by observation alone. However, this still remains an off-label use for thrombin, which was produced as a topical hemostatic agent. Bovine thrombin is generally used and is reconstituted in normal saline to a concentration of 1000 units/mL.
The procedure is performed by first anesthetizing the skin over the PSA with 1% lidocaine after preparation with a povidone-iodine (Betadine) solution. A 1-mL syringe is attached to a spinal needle (22 gauge) and introduced into the PSA under ultrasound guidance. Once the tip of the needle is identified within the sac, 0.1 to 0.2 mL of thrombin is injected. Care must be taken to ensure that the tip of the needle is directed away from the inflow neck of the PSA to decrease the potential for CFA or SFA embolization. Flow in the sac can be checked with color Doppler ultrasound, and if there is persistence, an additional 0.1 to 0.2 mL can be injected. A final ultrasound examination should be performed to confirm obliteration of flow within the PSA and a patent native artery. Distal pulses should be checked before and after the procedure, and a repeated duplex examination should be performed in 24 to 48 hours to confirm resolution of the PSA. As noted in Table 46-3, the recurrence rate is approximately 3%. The most significant complication of this procedure is thrombosis of the native artery or vein if thrombin is inadvertently injected into these vessels. Anaphylaxis has also been reported anecdotally, especially with bovine preparations of thrombin, but this complication is very rare.60
Conservative Management.
For PSAs that are small, observation alone is a reasonable treatment strategy. In a study by Kent et al,35 9 of 16 postcatheterization PSAs thrombosed spontaneously, with the majority of them being less than 1.8 cm in diameter. Toursarkissian et al29 monitored 82 patients with PSAs smaller than 3 cm in diameter, and 87% of these aneurysms thrombosed spontaneously at a mean of 23 days. Accordingly, reliable patients with PSAs smaller than 2 cm in diameter can be discharged home with close follow-up and frequent duplex ultrasound assessment. If there is an increase in the size of the PSA or symptoms develop, patients should undergo ultrasound-guided thrombin injection.
Surgical Management.
Despite the excellent results with thrombin injection, there still remains a role for surgical repair of PSAs (Box 46-2).61,62 Techniques of PSA repair include direct surgical repair and endovascular options. Surgical repair involves obtaining proximal and distal control before opening the aneurysm. For high lesions, exposure proximal to the inguinal ligament may be required for control of the external iliac artery. An alternative technique involves directly entering the PSA and controlling the arterial puncture site with digital pressure or placing occluding balloons into the proximal and distal vessels, then dissecting proximally and distally to gain control. The surrounding hematoma can be evacuated and the arteriotomy can be closed with interrupted polypropylene suture. Pitfalls to direct repair of PSAs include improperly identifying the arterial wall and closing the fascia only above the arterial defect. This will inevitably result in recurrent PSA or bleeding postoperatively. Like all groin explorations for vascular complications, PSA repair is associated with multiple potential complications. The most common adverse events related to PSA repair include postoperative bleeding, neuralgia, infection, and respiratory complications, although mortality remains low.63,64
Endovascular options for postangiography PSA exist, but they have not been adequately studied to date. Therefore, endovascular techniques are advocated only when other modalities of treatment of PSA have failed and patients are high-risk surgical candidates. Placement of covered stents, as discussed for AVF, is one option that is effective at covering the arteriotomy and obliterating flow into the aneurysm sac. However, placement of a stent across the femoral artery is not ideal, and there is a high risk for stent fracture and eventual occlusion. Alternatively, coil embolization can be performed by percutaneously accessing the PSA with a large-bore catheter and inserting coils directly into the sac under ultrasound guidance.65 This option might be useful in patients maintained with high-level anticoagulation or with a known allergy to thrombin.
Thrombosis
CFA thrombosis at the site of an endovascular procedure is a known complication of catheter-based interventions. Simple compression of the CFA after removal of the sheath can lead to thrombosis, especially in the presence of significant CFA atherosclerotic disease, previous groin reconstruction, or previously placed proximal anastomosis of a lower extremity bypass graft. The advent of closure devices for sealing the puncture site has potentially increased the incidence of thrombosis, and this is discussed in detail later in the chapter.
Thrombosis usually becomes apparent after the sheath has been removed and manual compression has been completed. The diminished flow through the CFA coupled with either a diseased SFA or injured intima from percutaneous access results in thrombosis of the lumen. Clot can then propagate to involve the profunda or iliac arteries and give rise to profound ischemia. Conversely, thrombus can develop on the sheath during the procedure or at the arteriotomy site, and on release of manual compression, it can embolize to the runoff bed. Acute ischemia requiring urgent intervention may develop, or patients may exhibit new-onset claudication or pain at rest. Treatment consists of groin exploration with femoral endarterectomy, patch angioplasty, and thromboembolectomy. Alternatively, patients may benefit from endovascular interventions, including mechanical thrombectomy, thrombolysis, and atherectomy, to remove or to dissolve the obstruction. As with all endovascular procedures, it is important to adequately assess the distal pulses and to document examination findings before beginning the procedure and then to compare those results with the results after the procedure and sheath removal to determine if there has been a change in the patient’s vascular examination findings.
Axillary and Brachial Access Complications
Although most peripheral interventions are performed through the groin, severe atherosclerotic disease of the femoral or iliac vessels can often make this approach difficult. As an alternative, the brachial and axillary arteries have demonstrated adequacy as access vessels for lower extremity, visceral, or carotid interventions.66,67 However, cannulation of the upper extremity vessels is not without risk. Complications of upper extremity access are similar to those associated with femoral artery access. In a large retrospective review of 2250 brachial artery catheterizations for lower extremity diagnostic angiography, Chatziioannou et al68 were successful in achieving brachial cannulation in more than 99% of cases. The major complication rate was just 0.44%; complications included PSA (0.09%), thrombosis (0.09%), hematoma (0.22%), and dissection (0.04%), and only three patients with these complications required surgical intervention. Minor complications were more prominent, however, the most common being ecchymosis, which was present in close to 14% of patients. All of these angiograms were diagnostic, and therefore only a 4F sheath was used; higher complication rates are more prevalent with interventions that necessitate the use of larger sheaths. In a cardiac catheterization study in which up to 7F sheaths were used, the major complication rate was approximately 5%.69 In evaluating the transaxillary approach, Chitwood et al70 found a 2.3% incidence of complications, and the most common was nerve injury. Risk factors identified for axillary artery injury included female gender, hypertension, periprocedural anticoagulation, thrombolysis, and prolonged (>90 minutes) procedures.
Nerve Injury
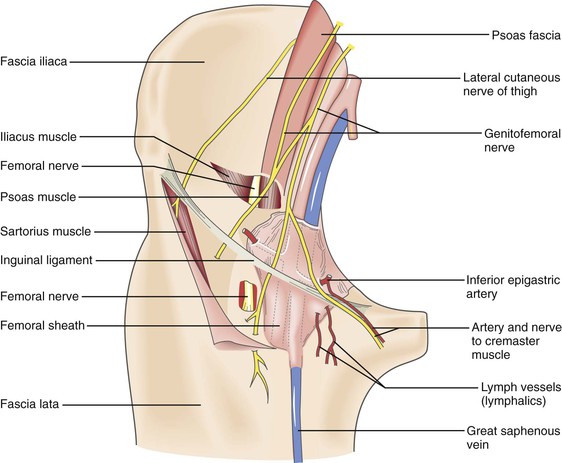
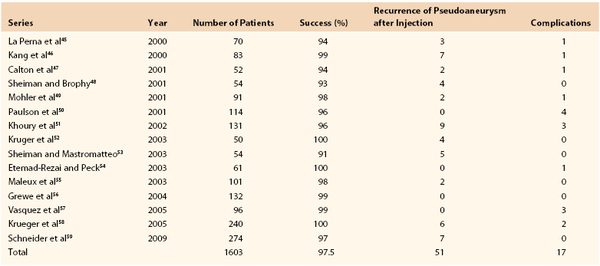
It is important to maintain high vigilance for nerve injury with transbrachial or transaxillary access because of the potential for permanent devastating disability of the upper extremity. Compared with the groin, in which the incidence of nerve injury from percutaneous procedures is extremely low (0.2%), brachial access can be associated with clinically significant neuropathy of the various terminal nerves of the infraclavicular brachial plexus at an incidence ranging between 0.4% and 12.7%.71–73 This neuropathy probably occurs because anatomically the median nerve and brachial artery are in close association and travel within the medial brachial fascial compartment (Fig. 46-6). There are three main mechanisms by which an injury can occur: hematoma within the fascia, causing compression of the nerve bundle; direct nerve damage by the needle or sheath; and ischemia of the nerve secondary to arterial thrombosis.23 Patients complain of pain radiating down the arm with associated muscle weakness and paresthesias. The most common and treatable cause of neurologic dysfunction is compression from an axillary sheath hematoma causing medial brachial fascial compartment syndrome. The clinical finding of hand weakness can, however, occur without evidence of a puncture site hematoma and in the setting of a palpable radial and ulnar pulse. Surgery is mandated for nerve compression symptoms and should be undertaken as soon as possible to minimize functional loss. It involves decompression of the fascial compartment with exposure of the brachial artery and repair of the arteriotomy.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree