Lipoprotein(a) (Lp[a]) may represent an independent risk factor for peripheral arterial disease of the lower limbs (LL-PAD), but prospective data are scant. We estimated the association between baseline Lp(a) with prevalent and incident LL-PAD in older subjects from the InCHIANTI Study. LL-PAD, defined as an ankle–brachial index <0.90, was assessed at baseline and over a 6-year follow-up in a sample of 1,002 Italian subjects 60 to 96 years of age. Plasma Lp(a) and potential traditional and novel cardiovascular risk factors (including a score based on relevant inflammatory markers) were entered in multivariable models to assess their association with prevalent and incident LL-PAD. At baseline, Lp(a) concentration was directly related to the number of increased inflammatory markers (p <0.05). There were 125 (12.5%) prevalent cases of LL-PAD and 57 (8.3%) incident cases. After adjustment for potential confounders, participants in the highest quartile of the Lp(a) distribution (≥32.9 mg/dl) were more likely to have LL-PAD compared to those in the lowest quartile (odds ratio [OR] 1.83, 95% confidence interval [CI] 1.01 to 3.33). The association was stronger (OR 3.80, 95% CI 1.50 to 9.61) if LL-PAD was defined by harder criteria, namely an ankle–brachial index <0.70. Compared to subjects in the lowest Lp(a) quartile, those in the highest quartile showed a somewhat increased risk of incident LL-PAD (lowest quartile 7.7%, highest quartile 10.8%), but the association was not statistically significant (OR 1.52, 95% CI 0.71 to 3.22). In conclusion, Lp(a) is an independent LL-PAD correlate in the cross-sectional evaluation, but further prospective studies in larger populations, with longer follow-up and more definite LL-PAD ranking, might be needed to establish a longitudinal association.
Several epidemiologic studies have suggested that plasma lipoprotein(a) (Lp[a]) is a strong risk factor for coronary heart disease and stroke, whereas fewer investigations have assessed the risk for peripheral arterial disease of the lower limbs (LL-PAD), yielding controversial results. Differences in Lp(a) plasma levels between populations suggest ethnic differences in the control of Lp(a) concentration, which may imply potentially different prognostic values; although LL-PAD has been investigated in Italy, only 1 population-based study examined Lp(a) and the risk of LL-PAD. A pathophysiologic link between increased Lp(a) levels and LL-PAD is plausible because this molecule has atherogenic and thrombogenic properties. Lp(a) contributes to foam cell formation because it is prone to oxidative modification, and when oxidized, it is taken up by the scavenger receptor pathway ; further, apolipoprotein(a) (apo[a]) has structural homology with plasminogen, suggesting that Lp(a) may exert antifibrinolytic properties. Prevalence of LL-PAD increases with age and older patients with LL-PAD have faster functional decrease, greater disability, and higher mortality compared to those without LL-PAD. Identifying risk factors for LL-PAD onset may disclose new opportunities for prevention and cure, thus contributing to a decrease in the burden of morbidity and functional consequences of LL-PAD in older patients. Using data from a population-based study of older Italian patients, we investigated the relation of baseline plasma Lp(a) levels with prevalent and incident LL-PAD.
Methods
The Invecchiare in Chianti (InCHIANTI) study is a prospective, population-based study of randomly selected older residents living in 2 cities in the Chianti area, Tuscany, Italy. The study was designed by the Laboratory of Clinical Epidemiology of the Italian Research Council of Aging (Florence, Italy) to identify risk factors for late-life disability, as previously described. Briefly, participants were selected from the city registries of Greve in Chianti and Bagno a Ripoli using a multistage sampling method. In 1998, 1,453 residents randomly selected from the population agreed to participate in the project (91.6% response rate). The Italian National Research Council on Aging ethical committee ratified the study protocol and participants provided written consent to participate. The present analysis was performed in 1,002 subjects ≥60 years of age, with an ankle–brachial index (ABI) <1.5 and plasma Lp(a) measured at baseline. Of the original 1,453 participants enrolled in the study, 250 were excluded because they were <60 years old, 3 because of an ABI >1.5, 182 because they had no ABI assessment at baseline, and 16 had missing Lp(a) values. Longitudinal analysis was limited to 686 participants without prevalent LL-PAD at baseline and with ≥1 valid ABI measurement over follow-up: 125 were excluded because of prevalent LL-PAD, 47 because they had died, and 144 because they did not have ABI assessment at follow-up. Observations lost to follow-up (n = 191) were significantly more likely to be older, to be men, and to have higher levels of inflammatory markers; they had similar baseline levels of Lp(a) and similar ABI scores.
ABI was measured during the clinical test session with a hand-held Doppler stethoscope (Parks model 41-A, Parks Medical Electronics, Inc., Aloha, Oregon). As previously described, systolic pressures were measured 2 times in the right brachial artery and 2 times in each posterior tibial artery. The highest pressure in each set of measurements was used to calculate the ABI score by dividing the lower of the 2 systolic pressures for each leg by the brachial artery pressure. ABI was measured at baseline and LL-PAD was defined as an ABI <0.90 and absence of LL-PAD as an ABI from 0.90 to 1.50. Patients were excluded if their ABI >1.50, indicating poorly compressible leg arteries and inability to gauge arterial perfusion accurately. An ABI <0.90 is consistent with LL-PAD. Participants were revaluated for ABI over follow-up at 3 and 6 years. Incident LL-PAD cases were defined as an ABI <0.90 at any follow-up visit in those without prevalent LL-PAD (ABI <0.90 at baseline).
Blood samples were obtained from participants after a 12-hour fast. Aliquots of serum and plasma were stored at −80°C and were not thawed until analyzed. Lp(a) concentration was evaluated by measuring apo(a) from frozen plasma by an enzyme-linked immunosorbent assay (Mercodia, Uppsala, Sweden) where apo(a) 1 U is approximately equal to Lp(a) 0.7 mg (Mercodia Manual); the results are expressed as milligrams per deciliter. This assay is very sensitive and highly specific and produces no measurable cross-reactivity with plasminogen and apolipoprotein B; in addition, it minimizes the possible interference of heterogeneity in apo(a) isoforms in the results; the detection limit is 0.0035 mg/dl; the overall coefficient of variation for Lp(a) measurements in this study was 6.6%. High-density lipoprotein cholesterol, total cholesterol, and triglycerides were determined using commercial enzymatic tests (Roche Diagnostics, Mannheim, Germany). We used the Friedwald equation to calculate low-density lipoprotein cholesterol concentration. Oxidized low-density lipoprotein was measured by enzyme-linked immunosorbent assay (Mercodia).
Cigarette-smoking behavior was assessed through survey questions. Daily alcohol (grams per day) and total energy intake (kilocalories per kilogram per day) were estimated by the European Prospective Investigation into Cancer and Nutrition Food Frequency Questionnaire. Weight and height were measured using objective standard techniques and used to calculate body mass index (kilograms per meter squared). Physical activity during the year before the interview was assessed through an interviewer-administered questionnaire as previously described. Responses were coded on an ordinal scale from 1 to 7. A score of 1 indicated no physical activity (bedrest) and a score of 6 indicated intense physical activity (i.e., a sport activity) performed several times per week. A score of 7 denoted participants who engaged in intensive and prolonged physical activity (≥5 km of walking per day occurring ≥5 times per week) consistently over the previous 5 years.
The presence of specific medical conditions was established using standardized criteria that combined information from self-reported history, medical records, and clinical medical examination. Participants were also asked to report any medication taken in the previous 2 weeks. Diagnostic algorithms were modified versions of those created for the Women’s Health and Aging Study. Assessed diseases were coronary heart disease (angina and acute myocardial infarction), stroke (and/or transient ischemic attack), hypertension, and diabetes. Serum creatinine and urinary creatinine from the 24-hour urine collection were measured using a modified Jaffe method and used to calculate creatinine clearance as a measurement of glomerular filtration rate.
Based on the results of previous work of this group, 4 inflammatory markers were considered for this project: interleukin (IL)-6, IL-1 receptor antagonist, fibrinogen and C-reactive protein. IL-6 and IL-1 receptor antagonist were measured in duplicate by high-sensitivity enzyme-linked immunoabsorbent assays (Biosource, Camarillo, California). Coefficients of variation were 4.5% for IL-1 receptor antagonist and 7% for IL-6; C-reactive protein was measured in duplicate using an enzyme-linked immunoabsorbent assay and colorimetric competitive immunoassay (interassay coefficient of variation 5%). Plasma fibrinogen levels were automatically determined using a commercially available STA Compact fibrinogen assay (Diagnostic Stago, Roche Diagnostics GmbH, Mannheim, Germany) according to the Clauss method using a Stago Boehringer Mannheim Analyzer (Boehringer Mannheim, Mannheim, Germany). The reference range used by this laboratory is 150 to 400 mg/dl.
Variables are reported as means ± SDs, median and interquartile range (quartiles 1 to 3), or percentages. Comparisons across Lp(a) quartiles were performed using analysis of variance, chi-square test, or Kruskal–Wallis nonparametric test for variables with skewed distribution. An ordinal score was created that summarized the intensity of the proinflammatory state across multiple markers. The score (range 0 to 4) consisted of the number of inflammatory markers in the upper tertiles, among the markers previously identified as independent correlates of ABI, namely C-reactive protein (>4.2 mg/dl), IL-6 (>1.8 pg/ml), IL-1 receptor antagonist (>162.7 pg/ml), and fibrinogen (>376 mg/dl). Multivariable logistic regression analysis was then used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the cross-sectional association between Lp(a) concentration and LL-PAD (ABI <0.9) or LL-PAD defined by more stringent criteria (ABI <0.7) approximating the median ABI value (0.72) in participants with an ABI <0.9. Covariates hypothesized as potential confounders of the association between plasma Lp(a) and LL-PAD were progressively added to the initial model. Longitudinal analysis was limited to 686 participants without prevalent LL-PAD at baseline and with ≥1 valid ABI measurement over follow-up. Incident LL-PAD was defined as an ABI value <0.9 assessed at the 3- or 6-year clinic visit in participants with an ABI value ≥0.9 at baseline. We used logistic multivariable analyses to estimate the strength of the association between baseline Lp(a) categories and likelihood of incident LL-PAD, adjusting for potential confounders; baseline ABI was included in the model to account for regression to the mean. To investigate selective loss to follow-up, we performed a sensitivity analysis in which we included also those who were lost to follow-up (n = 191). Two converse options were examined, assuming that those lost to follow-up would have incident LL-PAD or that none of them would have the condition. All analyses were performed using STATA 9.2 (STATA Corp., College Station, Texas).
Results
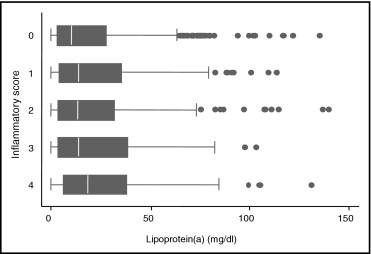
Mean age of participants was 73.7 years (range 60 to 95); 44.0% were men. Median Lp(a) concentration was 12.4 mg/dl (interquartile range 3.5 to 32.8). No differences in Lp(a) concentration were found according to age and gender. Prevalence of coronary heart disease and use of lipid-lowering medications were greater in those with the highest Lp(a) concentration. Participants in the highest Lp(a) quartile had the highest levels of total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol. No significant differences were found in triglycerides and oxidized low-density lipoprotein cholesterol according to Lp(a) quartiles ( Table 1 ). Of the inflammatory markers considered, only fibrinogen was significantly associated to Lp(a) concentration in univariate analysis; nevertheless, we found a direct significant association between Lp(a) plasma levels and the ordinal score summarizing the intensity of the proinflammatory state ( Figure 1 ).
| Characteristics | Lp(a) Quartiles (mg/dl) (range) | p Value ⁎ | |||
|---|---|---|---|---|---|
| 1 (0–3.5) (n = 251) | 2 (3.6–12.3) (n = 250) | 3 (12.4–32.8) (n = 249) | 4 (32.9–175.9) (n = 252) | ||
| Age (years) | 74.0 ± 7.3 | 74.1 ± 7.6 | 73.3 ± 6.7 | 74.3 ± 7.2 | 0.432 |
| Men | 43.8% | 44.8% | 43.0% | 44.4% | 0.978 |
| Smokers | |||||
| Former | 30.5% | 27.1% | 24.5% | 26.2% | |
| Current | 15.1% | 13.2% | 15.7% | 13.1% | 0.787 |
| Alcohol consumption (g/day) | |||||
| 0–20 | 47.0% | 44.0% | 47.0% | 46.0% | |
| >20 | 24.7% | 28.8% | 25.4% | 25.2% | 0.972 |
| Physical activity level scale (1–7, 7 = best) | 3.2 ± 0.98 | 3.2 ± 0.95 | 3.3 ± 0.88 | 3.2 ± 1.02 | 0.516 |
| Body mass index (kg/m 2 ) | 27.2 ± 4.0 | 27.8 ± 4.0 | 27.4 ± 4.2 | 27.6 ± 3.8 | 0.293 |
| Hypertension | 62.2% | 60.0% | 62.7% | 62.3% | 0.928 |
| Positive Rose Questionnaire | 14.4% | 24.4% | 28.9% | 32.2% | 0.080 |
| Diabetes mellitus | 13.9% | 11.6% | 12.1% | 9.9% | 0.578 |
| Coronary heart disease (angina or myocardial infarction) | 8.0% | 8.8% | 10.8% | 15.1% | 0.046 |
| Stroke or transient ischemic attack (%) | 7.2% | 4.8% | 6.4% | 7.9% | 0.533 |
| Statins therapy | 0.4% | 2.0% | 3.6% | 7.9% | <0.001 |
| Total cholesterol (mg/dl) | 215.3 ± 41.4 | 215.3 ± 38.4 | 217.6 ± 36.6 | 227.0 ± 38.2 | 0.0015 |
| Low-density lipoprotein cholesterol (mg/dl) | 132.6 ± 36.7 | 135.0 ± 33.2 | 137.3 ± 31.3 | 144.2 ± 32.7 | <0.001 |
| High-density lipoprotein cholesterol (mg/dl) | 56.3 ± 16.7 | 54.1 ± 14.2 | 55.0 ± 14.4 | 57.8 ± 14.3 | 0.029 |
| Oxidized low-density lipoprotein cholesterol (U/L) | 41.4 ± 13.3 | 42.9 ± 12.6 | 42.7 ± 11.8 | 42.9 ± 12.9 | 0.435 |
| Triglycerides (mg/dl) | 105 (81–161) | 117 (84–161) | 112 (85–148) | 108 (88–146) | 0.709 |
| Creatinine clearance (ml/min) | 77.5 ± 27.1 | 78.8 ± 25.8 | 76.9 ± 25.2 | 75.2 ± 24.9 | 0.487 |
| High-sensitivity C-reactive protein (μg/ml) | 2.44 (1.11–4.92) | 2.36 (1.27–5.51) | 2.82 (1.36–5.83) | 2.99 (1.53–5.57) | 0.151 |
| Interleukin-6 (pg/ml) | 1.38 (0.78–2.01) | 1.47 (0.88–2.36) | 1.44 (0.84–2.09) | 1.38 (0.86–2.27) | 0.629 |
| Fibrinogen (mg/dl) | 345 (308–390) | 340 (302–390) | 345 (312–395) | 360 (318–413) | 0.007 |
| Interleukin-1 receptor antagonist (pg/ml) | 127 (95–175) | 136 (101–192) | 137 (93–190) | 134 (95–180) | 0.472 |
| Homocysteine (μmol/L) | 14.8 (12.2–17.5) | 14.6 (12.0–17.3) | 14.4 (11.9–17.9) | 13.5 (11.7–16.5) | 0.176 |
| Estimated creatinine clearance (ml/min) | 64.9 ± 20.8 | 65.6 ± 19.1 | 64.4 ± 18.4 | 63.9 ± 18.3 | 0.784 |
⁎ Analysis of variance, chi-square test, or Kruskal–Wallis nonparametric test for variables with skewed distribution.
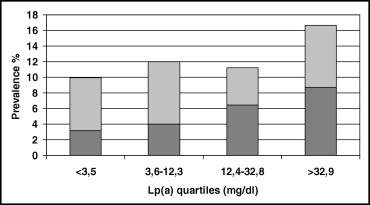
As depicted in Figure 2 , there was a stepwise increase in the likelihood of prevalent LL-PAD across quartiles of Lp(a) distribution (p for linear trend = 0.038). The association was stronger when analysis was limited to participants with an ABI <0.7, a proxy for more severe disease (n = 66, p for linear trend = 0.004), who had higher Lp(a) plasma levels (median 25.7 ml/dl) than those with ABIs 0.7 to <0.9 and ≥0.9 (median Lp[a] 11.8 and 12.1 mg/dl, respectively, p = 0.005 Kruskal–Wallis test). These findings were confirmed by multivariable logistic regression analysis estimating the likelihood of prevalent LL-PAD as a function of Lp(a) distribution ( Table 2 ). After adjustment for age, gender, smoking, and alcohol intake (model 1), subjects in the highest quartile of the Lp(a) distribution had a twofold higher probability of being affected by LL-PAD compared to those in the lowest quartile. Further adjustment for body mass index, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, diabetes, and hypertension did not modify the association (model 2), whereas a mild decrease was observed after adjusting for inflammation score (model 3), but the strength of the association remained statistically significant. In analysis limited to an ABI <0.70, there was a clear dose–response relation across quartiles of Lp(a). After full adjustment for potential confounders and compared to subjects in the lowest quartile (model 3), those in the third quartile were >2 times as likely to have LL-PAD, and participants in the highest quartile had almost a fourfold probability of LL-PAD (OR 3.8, 95% CI 1.5 to 9.6, p = 0.005). Further adjustment for statin use and estimated creatinine clearance did not modify the results. No significant interactions between Lp(a) concentration and other cardiovascular risk factors were observed.