Obstructive and central sleep apneas are treatable disorders, which contribute to cardiovascular morbidity in older adults. Younger adults with Marfan syndrome may also be at risk for sleep apnea, but the relation between cardiovascular complications and sleep apnea is unknown. We used MiniScreen8 portable monitoring devices for polygraphy in 68 consecutive adults with Marfan syndrome (33 men, 35 women, 41 ± 14 years old) to investigate frequency of sleep apnea and its relation to cardiovascular morbidity. The apnea–hypopnea index (AHI) was 6 to 15/hour in 14 subjects (mild sleep apnea, 21%), and AHI was >15/hour in 7 subjects (moderate or severe sleep apnea, 10%). Among established risk factors for sleep apnea, only older age (Spearman rho = 0.35, p = 0.004) and body mass index (rho = 0.26, p = 0.03) were associated with increased AHI. Of all cases of apnea, 12 ± 27 were obstructive, 11 ± 25 central, and 3 ± 9 mixed. AHI was associated with decreased left ventricular ejection fraction (rho = −0.33, p = 0.01), increased N-terminal pro–brain natriuretic peptide levels (rho = 0.35, p = 0.004), enlarged descending aortic diameters (rho = 0.44, p = 0.001), atrial fibrillation (phi = 0.43, p = 0.002), and mitral valve surgery (phi = 0.34, p = 0.02). Of these, left ventricular ejection fraction, N-terminal pro–brain natriuretic peptide levels, atrial fibrillation, and mitral valve surgery were associated with AHI independently of age and body mass index. We found similar associations with oxygen desaturation index. In conclusion, sleep apnea exhibits increased frequency in Marfan syndrome and is not predicted by classic risk factors. Obstructive and central sleep apneas may relate to cardiovascular disease variables.
Epidemiologic studies have documented an association between obstructive sleep apnea (OSA) and cardiovascular diseases such as arterial hypertension, coronary artery disease, arrhythmias, heart failure, stroke, and aortic dissection, whereas central sleep apnea (CSA) has predominantly been related to heart failure. Continuous positive airway pressure is an effective treatment, which has been shown to decrease the risk of cardiovascular disease. Recently, 2 centers reported an increased frequency of OSA in Marfan syndrome. Marfan syndrome is an autosomal, dominantly inherited disease of the connective tissue with manifestations in the eyes, lungs, dura, and skeleton, which results from mutations in the gene coding for fibrillin-1, FBN1 . Untreated patients with Marfan syndrome die prematurely due to dissection and rupture of the aorta. Despite effective treatment of aortic root disease, cardiovascular morbidity remains high due to arrhythmia, heart failure, complications at distal sites of the aortic vessel, and heart valve dysfunction. We performed the present study to investigate, first, whether sleep apnea is present in a relevant subset of unselected adults with Marfan syndrome, and second, whether sleep apnea relates to cardiovascular morbidity.
Methods
We used portable 8-channel monitoring devices for ambulatory, unattended respiratory polygraphy. All patients were >17 years of age, all presented to our clinic within a period of 1 year, all lived within the Hamburg metropolitan area, and all were evaluated according to the Ghent nosology. We did not enroll patients in whom the Marfan syndrome had been excluded, pregnant women, patients with blindness, and patients on continuous positive airway pressure therapy. We continued all medical regimens. We evaluated 106 consecutive outpatients. Of these, 30 patients did not fulfill criteria of Marfan syndrome, 2 were on continuous positive airway pressure treatment, 1 was blind, 1 did not complete our diagnostic program, 2 declined to participate in our study, and 2 had inadequate MiniScreen8 measurements. The remaining 68 patients with Marfan syndrome constituted our study group, with 33 men and 35 women with a mean age of 41 ± 14 years (range 18 to 70; Table 1 ).
| Variable | All Patients (n = 68) | Range | AHI | ODI | ||
|---|---|---|---|---|---|---|
| Correlation Coefficient ⁎ | p Value † | Correlation Coefficient ⁎ | p Value † | |||
| Male gender | 33 (49%) | 0.29 | 0.06 | 0.26 | 0.11 | |
| Age (years) | 41 ± 14 | 18–70 | 0.35 | 0.004 | 0.46 | <0.001 |
| Body weight (kg) | 79 ± 20 | 47–127 | 0.24 | 0.05 | 0.36 | 0.003 |
| Body height (m) | 1.85 ± 0.2 | 1.52–2.08 | 0.01 | 0.9 | 0.09 | 0.5 |
| Body mass index (kg/m 2 ) | 22 ± 4 | 15–32 | 0.26 | 0.03 | 0.38 | 0.002 |
| Body surface area (m 2 ) | 2 ± 0.2 | 1.49–2.65 | 0.17 | 0.2 | 0.31 | 0.01 |
| Neck circumference (cm) | 37 ± 4 | 29–50 | 0.23 | 0.06 | 0.36 | 0.003 |
| Active smoker | 5 (8%) | 0.16 | 0.45 | 0.11 | 0.71 | |
| Blood glucose (mg/dl) | 93 ± 17 | 64–155 | 0.18 | 0.2 | 0.24 | 0.06 |
| Total cholesterol (mg/dl) | 185 ± 42 | 103–290 | 0.23 | 0.08 | 0.35 | 0.007 |
| High-density lipoprotein cholesterol (mg/dl) | 58 ± 18 | 27–104 | 0.05 | 0.7 | −0.03 | 0.8 |
| Low-density lipoprotein cholesterol (mg/dl) | 104 ± 35 | 45–191 | 0.11 | 0.4 | 0.26 | 0.05 |
| Triglyceride (mg/dl) | 123 ± 78 | 40–474 | 0.16 | 0.2 | 0.25 | 0.06 |
| Epworth Sleepiness Score >10 | 13 (19%) | 0.27 | 0.09 | 0.15 | . 46 | |
| Berlin questionnaire ≥2 | 17 (25%) | 0.16 | 0.42 | 0.27 | 0.08 | |
⁎ Spearman correlation coefficient (rho) for continuous variables. AHI and ODI were grouped 0 to 5, 6 to 15, and >15 to assess the phi coefficient for binary variables.
The Hamburg research ethics committee approved our protocol, which was in accordance with principles of the Declaration of Helsinki. All subjects gave written informed consent. We performed baseline clinical examinations including baseline serum examinations, blood pressure measurement, standard 12-lead electrocardiography, transthoracic echocardiography, and magnetic resonance angiography of the entire aorta within 24 hours of polygraphy in all patients. An experienced technician provided individual training on how to use the MiniScreen8 device and a standardized written and illustrated device instruction to all patients. We used MiniScreen8 type 3 devices (Heinen and Löwenstein, Bad Ems, Germany) in all patients. During sleep the devices record nasal flow with a pressure transducer system, oxygen saturation and pulse rate by finger oximetry, body position through a magnetic sensor, snoring sounds, and thoracic and abdominal movements through belts with pneumatic cushions for pressure measurement. All patients used the device for 1 full night at home. We performed automated analysis of downloaded physiological data within 2 days of polygraphy. We also reviewed all electronically stored data manually using EasyScreen Viewer 5.09 (Heinen und Löwenstein) jointly with a board-certified physician of sleep medicine. All investigators were blinded to medical history and other medical data. In 7 subjects with technically insufficient recordings we initiated a second polygraphy yielding adequate data in 5 subjects, and we excluded 2 subjects because they declined to repeat polygraphy.
We assessed body height using a wall-fixed height rule, body mass index, body surface area, and neck circumference measured at the level of the cricothyroid membrane. We considered active smoking with any inhaled intake of nicotine within ≤7 days before polygraphy and we measured fasting blood glucose levels and fasting lipid levels ≤24 hours of the study. We used the Epworth Sleepiness Scale questionnaire and the Berlin questionnaire to assess subjective sleepiness ( Table 1 ).
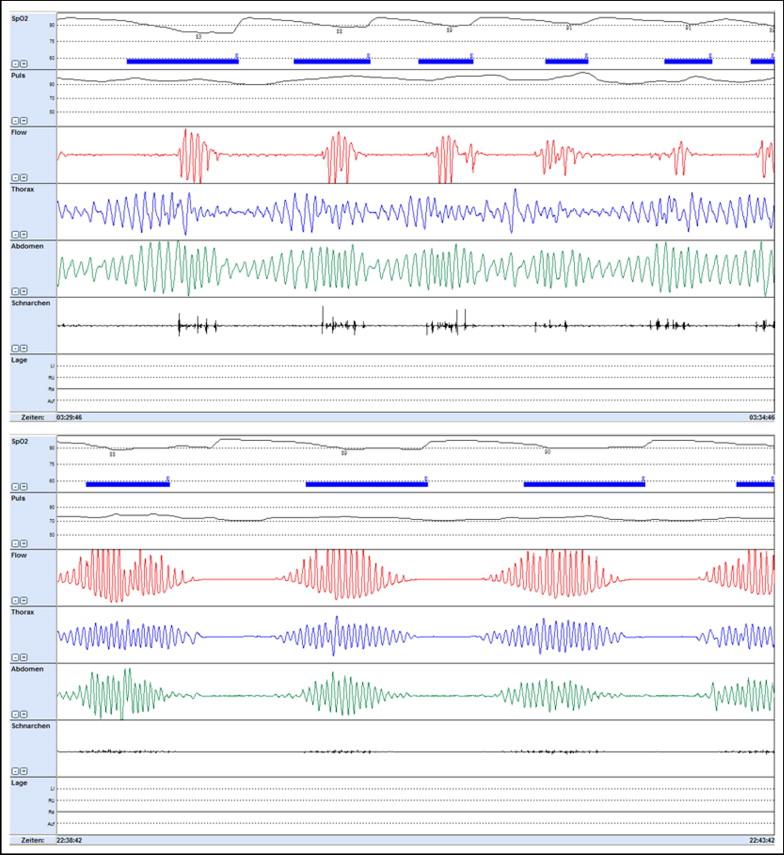
We presented heart rates and blood oxygen saturation as the mean during total recording time with documentation of lowest saturation and longest duration of desaturation. We defined an episode of apnea as the cessation of airflow lasting >10 seconds and hypopnea as a decrease in airflow of ≥50% lasting >10 seconds, associated with a decrease in oxygen saturation of ≥4%. We quantified severity of sleep apnea as the number of apneas–hypopneas per hour (apnea–hypopnea index [AHI]) and as the number of oxygen desaturations (oxygen desaturation index [ODI]) ≥4% per hour of study. We used established thresholds for AHI and ODI to classify a sleep-related breathing disorder as absent with ≤5 events per hour, as mild with 6 to 15 events per hour, as moderate with >15 events per hour, and as severe with >30 events per hour. We considered obstructive apnea as ongoing ventilatory efforts documented by thoracic and abdominal movement signals for >2 respiratory cycles. We defined apnea as central when apnea episodes presented without any ongoing ventilatory efforts ( Figure 1 ), and mixed apnea when airflow continued to be absent despite resumption of respiratory efforts in an apnea episode with initial cessation of respiratory efforts. We assessed snoring severity as the number of snoring events per hour ( Table 2 ).
| Variable | All Patients (n = 68) | Range | AHI | ODI | ||
|---|---|---|---|---|---|---|
| Correlation Coefficient ⁎ | p Value † | Correlation Coefficient ⁎ | p Value † | |||
| Total recording time (minutes) | 601 ± 122 | 409–803 | 0 | 0.9 | −0.07 | 0.6 |
| Mean heart rate (beats/min) | 65 ± 11 | 45–103 | 0.14 | 0.3 | 0.35 | 0.004 |
| Oxyhemoglobin saturation | ||||||
| Mean (%) | 92 ± 12 | 88–96 | −0.44 | <0.001 | −0.51 | <0.001 |
| Lowest (%) | 80 ± 19 | 66–94 | −0.35 | 0.004 | −0.51 | <0.001 |
| Longest desaturation (minutes) | 1.1 ± 0.9 | 0.12–3.43 | 0.47 | <0.001 | 0.64 | <0.001 |
| Episodes of apnea (number) ‡ | ||||||
| Total | 27 ± 49 | 0–289 | 0.63 | <0.001 | 0.24 | 0.05 |
| Obstructive | 12 ± 27 | 0–163 | 0.49 | <0.001 | −0.04 | 0.6 |
| Central | 11 ± 25 | 0–145 | 0.34 | 0.006 | 0.07 | 0.6 |
| Mixed | 3 ± 9 | 0–66 | 0.47 | <0.001 | 0.25 | 0.048 |
| Longest apnea (seconds) | 24 ± 17 | 11–87 | 0.59 | <0.001 | 0.27 | 0.03 |
| Episodes of hypopnea (number) | 18 ± 24 | 0–109 | 0.76 | <0.001 | 0.88 | <0.001 |
| Longest hypopnea (seconds) | 36 ± 33 | 11–181 | 0.62 | <0.001 | 0.58 | <0.001 |
| Snore index (per hour) | 67 ± 126 | 0–111 | 0.33 | 0.008 | 0.44 | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


