Diagnostic coronary balloon occlusion (CBO) is mandatory for collateral function assessment, during angioscopy and optical coherence imaging, and when using certain coronary protection devices against emboli. Thus far, the safety of diagnostic CBO regarding procedural and long-term complications in normal coronary arteries has not been studied. In 316 patients, diagnostic CBO was performed for collateral function measurement in 426 angiographically normal vessels. The angioplasty balloon was inflated for 60 to 120 seconds using inflation pressures of 1 to 3 atm, followed by control angiography during and after CBO. Patients were divided into groups with entirely normal (n = 133) and partially normal (n = 183) vessels. Primary end points were procedural and long-term complications. De novo stenosis development was assessed by quantitative coronary angiography in 35% of the patients. Secondary end points were cardiac events at 5 years of follow-up. Procedural complications occurred in 1 patient (0.2%). In 150 repeat angiographic procedures in 92 patients (follow-up duration 10 ± 15 months), quantitative coronary angiography revealed no difference in percentage diameter narrowing between baseline and follow-up (4.1% vs 3.9%, p = 0.69). During follow-up periods of 14 and 72 months, respectively, a new stenotic lesion was detected in 1 patient in each group (1.3%). Major cardiac events and percutaneous coronary intervention for stable angina were less frequent in the group with entirely normal than with partially normal vessels (0.8% vs 5.5%, p = 0.02, and 0.8% vs 18%, p <0.0001). In conclusion, low–inflation pressure diagnostic CBO in angiographically normal coronary arteries bears a minimal risk for procedural and long-term complications and can therefore be regarded as a safe procedure.
During percutaneous coronary intervention (PCI), the atherosclerotic stenosis is dilated by structural disintegration of all 3 vessel wall layers, with subsequent scarring, radial stabilization, and neoendothelialization. In contrast, low–inflation pressure diagnostic coronary balloon occlusion (CBO) is thought not to injure any vessel wall layer ( Figure 1 ). For collateral function measurements, optical coherence tomography, and the use of certain coronary protection devices against emboli and angioscopy, diagnostic CBO is mandatory. To minimize the risk for dislocating atheromatous debris from the vessel wall, diagnostic CBO is preferably performed in vessel segments that appear angiographically normal. Before the use of stents, restenosis of stenotic arteries occurred in 30% to 50% of cases <6 months after PCI. In contrast, diagnostic CBO is performed using very low inflation pressures of 1 to 3 atm, just sufficient to block anterograde blood flow. For complete occlusion, however, the surface of the balloon must be in contact with the endothelial layer. This may be potentially harmful and result in early or late complications, such as thrombus formation or de novo stenosis, respectively. Normally, the integrity of the endothelial layer is maintained by endothelial progenitor cells and mitosis of endothelial cells. So far, there are no data on the safety of diagnostic normal-vessel CBO after follow-up periods >6 months, so the purpose of the present study was to assess the complication rate related to such a procedure.
Methods
From 1996 to 2008, 316 patients (mean age 61 ± 11 years; 243 men, 73 women) who were referred for diagnostic coronary angiography because of suspected coronary artery disease were included in the study if they presented with ≥1 coronary artery with normal angiographic appearance. All patients underwent diagnostic CBO for collateral function assessment in a total of 426 vessels. Patients with acute coronary syndromes, valvular heart disease, left ventricular ejection fractions <40%, previous coronary artery bypass surgery, abnormal conduction on electrocardiography, electrocardiographic signs of infarction in the studied region, chronic lung disease, or renal insufficiency were excluded. Subsequently, patients were divided into a group without any angiographic stenotic lesions (termed “entirely normal coronary arteries”; n = 141) and a group with angiographic stenotic lesions in coronary arteries other than those that underwent diagnostic CBO (termed “partially normal coronary arteries”; n = 285).
Patients underwent diagnostic left-sided cardiac catheterization via the right femoral artery. Before coronary angiography, 2 puffs of oral isosorbide dinitrate were given. Biplane left ventriculography was performed after the completion of coronary angiography, and left ventricular end-diastolic pressure was determined. Aortic pressure (P Ao ) was recorded using a 6Fr guiding catheter. On the basis of angiography, diagnosis of entirely or partially normal vessels was established and supplemented by fractional flow reserve measurement.
Standard image acquisition was performed using ≥2 angiographic projections at the site of diagnostic CBO, with repetition of identical views at the time of follow-up angiography. With the contrast-filled injection catheter as the calibration source, biplane quantitative coronary analysis was performed off-line using a validated automated software (QAngio XA 7/1/2027; Medis Medical Imaging Systems, Leiden, The Netherlands). Angiographic percentage diameter stenosis was defined as (1 − [minimal luminal diameter/reference vessel diameter]) × 100. Hemodynamically relevant stenosis was defined as ≥50% diameter luminal narrowing.
In the present study, diagnostic CBO was performed for the purpose of collateral function measurement after angiographic verification of normal vessel structure. After the administration of heparin 5,000 IU, inflation of an angioplasty balloon (varying from 8 to 20 mm in length) was performed for 60 to a maximum of 120 seconds at low inflation pressures of 1 to 3 atm. During inflation, angiography was performed to ensure complete occlusion ( Figure 1 ). An intracoronary electrocardiogram was recorded during CBO in every patient, and the incidence of pectanginal chest pain was noted. After removal of the angioplasty catheter at the end of measurements, further angiography to assess vascular structure with respect to potential spasm, thrombi, wall irregularities, dissections, or flow alteration was performed. As a preventive measure for potential coronary spasm after diagnostic CBO, all patients received a transdermal nitroglycerin patch for the following 12 hours per protocol.
In the absence of chronic total occlusion, diagnostic CBO is necessary for collateral function measurement: simultaneous coronary occlusive pressure (i.e., the pressure distal to the site of a complete balloon occlusion), P Ao , and central venous pressure are obtained. The collateral flow index is calculated as (coronary occlusive pressure − central venous pressure)/(P ao − central venous pressure). Coronary occlusive pressure was recorded using a 0.014-inch sensor-tipped pressure guidewire (Certus PressureWire; Radi Medical Systems, Uppsala, Sweden).
After obtaining written informed consent to participate in a study of collateral function, coronary angiography was performed. When eligible, patients were included and diagnostic CBO was performed in stenotic and normal coronary arteries. Among a total of 1,318 collateral flow index measurements, 426 diagnostic CBOs were carried out in normal vessels. In a subgroup of 18 patients, repeat angiographic procedures were performed with diagnostic CBO for study reasons (only placebo cases included in the present analysis) or because of recurrent chest pain with suspected ischemia. With respect to the primary end point of the study (i.e., procedural and long-term vascular complications), all repeat angiograms were analyzed quantitatively at the site of diagnostic CBO, and percentage diameter stenosis was calculated by quantitative coronary angiography for comparison with baseline values. All investigations with CBO in normal vessels were approved by the institutional ethics committee.
Aside from the primary end point of vascular complications, clinical follow-up information was obtained by phone interrogations or by examination of patient records for the occurrence of all-cause death, cardiac death, acute myocardial infarction, and unstable and stable angina pectoris after study inclusion. Cardiac death was defined as any death with an immediate cardiac cause (myocardial infarction, low cardiac output failure, fatal arrhythmia) or unwitnessed death.
For comparison of continuous demographic, angiographic, and hemodynamic variables between the 2 groups, unpaired Student’s t tests were used. For comparison of quantitative angiographic and functional results between baseline and follow-up examinations, paired Student’s t tests was used. Categorical variables between the 2 populations were compared using chi-square tests. Data are expressed as mean ± SD. Statistical significance was defined as p <0.05.
Results
Among the 426 vessels with diagnostic CBO in 316 patients, 133 patients (42%) had angiographically entirely normal coronary arteries. The remaining 183 patients (58%) had ≥1 stenotic lesion in 1 or 2 coronary arteries (i.e., partially normal coronary arteries). Because of the dichotomization into entirely and partially normal coronary artery groups, there were by definition given differences between the 2 groups: patients with entirely normal coronary arteries were younger, had fewer cardiovascular risk factors, and had fewer cardiovascular medications than patients with partially normal vessels ( Table 1 ).
| Variable | Entirely Normal Vessels (141 Coronary Arteries in 133 Patients) | Partially Normal Vessels (285 Coronary Arteries in 183 Patients) | p Value |
|---|---|---|---|
| Age (years) | 59 ± 11 | 63 ± 11 | <0.0001 |
| Body mass index (kg/m 2 ) | 27.6 ± 6.0 | 27.6 ± 4.0 | 0.98 |
| Men | 98 (70%) | 232 (81%) | 0.006 |
| Hypertension | 70 (50%) | 175 (61%) | 0.022 |
| Smoking | 36 (26%) | 89 (31%) | 0.26 |
| Hypercholesterolemia | 68 (48%) | 213 (75%) | <0.0001 |
| Diabetes mellitus | 16 (11%) | 36 (13%) | 0.76 |
| Acetylsalicylic acid | 71 (50%) | 252 (88%) | <0.0001 |
| Clopidogrel | 1 (1%) | 51 (18) | <0.0001 |
| β blockers | 57 (40%) | 189 (66) | <0.0001 |
| Calcium channel blockers | 23 (16%) | 37 (13) | 0.38 |
| Nitrates | 18 (13%) | 56 (20) | 0.10 |
| Statins | 46 (33%) | 209 (73%) | <0.0001 |
| Angiotensin-converting enzyme inhibitors | 33 (23%) | 107 (38%) | 0.004 |
| Angiotensin receptor blockers | 11 (8%) | 37 (13%) | 0.142 |
| Diuretics | 29 (22%) | 82 (29%) | 0.08 |
Mean aortic blood pressure (93 ± 15 vs 92 ± 14 mm Hg, p = 0.232), left ventricular end-diastolic pressure (10.6 ± 4.6 vs 11.7 ± 6.0 mm Hg, p = 0.251), and collateral flow index (0.17 ± 0.09 vs 0.16 ± 0.09, p = 0.324) did not differ between the groups, whereas heart rate (75 ± 13 vs 70 ± 13 beats/min, p <0.0001), the left ventricular ejection fraction (75 ± 13% vs 70 ± 13%, p = 0.001), and fractional flow reserve (0.95 ± 0.04 vs 0.93 ± 0.06, p = 0.005) differed between the groups with entirely versus partially normal coronary arteries.
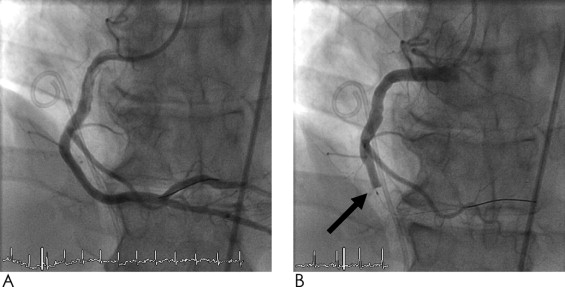
There were significant differences between the groups with regard to the distribution of vessels that underwent diagnostic CBO and to the number of stenotic lesions ( Table 2 ). The sites of CBO were equally distributed between the groups, with most diagnostic CBOs at proximal vessel sites. One acute complication occurred in a patient with entirely normal coronary arteries (i.e., acute thrombus formation during guidewire probing of the left circumflex coronary artery despite adequate application of heparin). The thrombus was visualized by intravascular ultrasound and thrombus aspiration and PCI were performed immediately, with excellent results ( Figure 2 ). In 303 of 426 CBOs (71%), patients experienced anginal chest pain during the procedures. ST-segment changes on intracoronary electrocardiography were detected in 78% of the cases. In all patients, CBO-related angina disappeared shortly after balloon deflation. In 150 of the 426 vessels (35%) in 92 patients, repeat angiography was performed either for study reasons (50%) or because of recurrent chest pain (50%) after a mean follow-up period of 10 ± 15 months. In patients with >1 repeat angiographic procedure, the latest was analyzed. The frequency of repeat angiography was different between the groups ( Table 2 ), because of participation in study protocols with scheduled follow-up angiography and recurrent ischemia related to vessels other than the CBO vessel: patients with entirely normal coronary arteries underwent repeat angiography significantly less often than those with partially normal vessels. Assessment by quantitative coronary angiography revealed no difference between baseline and follow-up percentage diameter stenosis (4.1% vs 3.9%, p = 0.69; Figure 3 ). After 14 and 72 months, respectively, a new stenosis at the site of previous diagnostic CBO in 1 patient in each group (1.3%) was found and treated by PCI. In 1 patient (PR), quantitative coronary angiography revealed a 20% diameter stenosis at baseline, which then progressed to 50% after 14 months ( Figure 3 ). In these 2 patients, the following balloon sizes, balloon lengths, and inflation pressures were used at baseline: in PR, 3.0 × 18 mm and 1 atm; in BP, 3.5 × 8 mm and 3 atm. The absolute sizes of the vessels at the site of diagnostic CBO were 3.39 mm (PR) and 4.04 mm (BP), respectively.
| Variable | Entirely Normal Vessels (141 Coronary Arteries in 133 Patients) | Partially Normal Vessels (285 Coronary Arteries in 183 Patients) | p Value |
|---|---|---|---|
| Baseline data | |||
| Vessel of diagnostic coronary balloon occlusion | |||
| Left anterior descending coronary artery | 69 (49%) | 92 (32%) | 0.0009 |
| Left circumflex coronary artery | 41 (29%) | 147 (52%) | <0.0001 |
| Right coronary artery | 31 (22%) | 46 (16%) | 0.14 |
| Number of stenotic lesions | |||
| 0 | 141 (100%) | 0 | <0.0001 |
| 1 | 0 | 185 (65%) | <0.0001 |
| 2 | 0 | 55 (19%) | <0.0001 |
| ≥3 | 0 | 45 (16%) | <0.0001 |
| Vessel segment of coronary balloon occlusion | |||
| Proximal | 130 (92%) | 269 (94%) | 0.38 |
| Midartery | 11 (8%) | 14 (5%) | 0.23 |
| Distal | 0 | 2 (1%) | 0.32 |
| Acute complications | 1 (0.7%) | 0 | 0.15 |
| Follow-up data | |||
| Repeat angiography after coronary balloon occlusion | 8 (6%) | 142 (50%) | <0.0001 |
| 1 time | 5 (4%) | 62 (22%) | <0.0001 |
| 2 times | 0 | 54 (19%) | <0.0001 |
| ≥3 times | 3 (2%) | 24 (8%) | 0.004 |
| Second coronary balloon occlusion at repeat angiography | 3/8 (38%) | 72/142 (51%) | 0.47 |
| Follow-up duration to repeat angiography (months) | 28.3 ± 21 | 8.9 ± 14 | <0.0001 |
| De novo stenosis at site of previous coronary balloon occlusion | 1 (0.7%) | 1 (0.4%) | 0.61 |
| Follow-up duration of cases with de novo stenosis (months) | 71.5 | 13.5 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


