Psoriasis is a chronic inflammatory disease associated with a variety of co-morbid conditions, including cardiovascular disease. Advancements in our understanding of the cellular and molecular mechanisms of psoriasis have led to a better understanding regarding its pathogenesis, which in turn has stimulated ongoing research to identify the underlying pathophysiology responsible for the increased risk of cardiovascular events associated with psoriasis. Although not yet fully elucidated, emerging evidence points to immune-mediated inflammation as a process that contributes to endothelial cell dysfunction, dyslipidemia, and atherosclerosis as key processes influencing cardiovascular disease in psoriasis. In particular, the dyslipidemia present in psoriasis may be associated with altered lipoprotein function and increased atherogenicity. Here, we review how the cytokine networks involved in lipoprotein metabolism and inflammation could impact on the cardiovascular disease risk for patients with psoriasis.
Psoriasis is a chronic, systemic, immune-mediated, inflammatory disorder with a prevalence in the United States in adults aged 20 years or older of 3.2% (95% CI 2.2% to 4.6%). Psoriasis and rheumatoid arthritis, another inflammatory disorder, are both characterized by a greater risk of cardiovascular disease than control patients or the general population. The complex interplay between cellular and inflammatory cytokine components may be partly responsible for mediating the increased risk of cardiovascular complications observed in patients with psoriasis. Here, we explore how the cytokine networks involved in lipoprotein metabolism and endothelial cell dysfunction and inflammation may impact on the risk of cardiovascular events for patients with psoriasis, to better understand the underlying processes and therapeutic implications.
Evidence of Increased Risk of Cardiovascular Co-Morbidities
Immune-mediated inflammatory diseases such as psoriasis are associated with a variety of co-morbidities, including cardiovascular complications. This risk is likely related to the widespread inflammation demonstrated in the large arteries of patients with psoriasis using [18F]-fluorodeoxyglucose positron emission tomography–computed tomography imaging ( Figure 1 ).
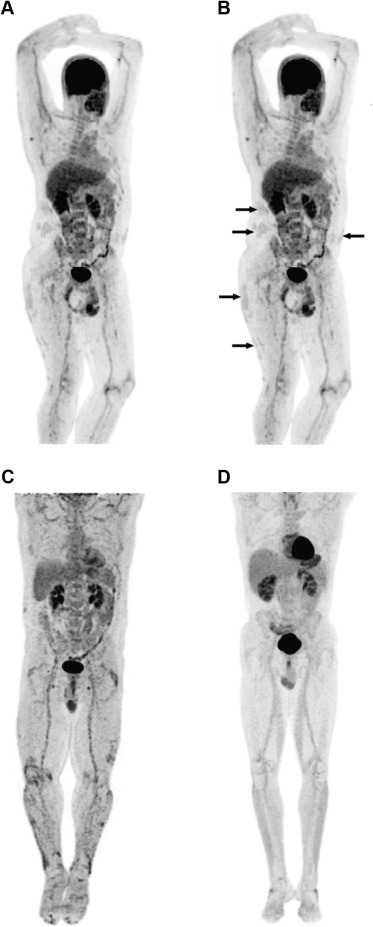
Psoriasis is frequently associated with co-morbidities that are also recognized risk factors for cardiovascular disease, including obesity, diabetes, dyslipidemia, and metabolic syndrome. However, studies have reported an independent association of psoriasis and increased risk of myocardial infarction, stroke, or hypertension, even after adjustment for known risk factors. Furthermore, an increased risk of cardiovascular events in patients with psoriasis is not universally accepted. A Dutch cohort study concluded that psoriasis was not a relevant risk factor for ischemic heart disease, whereas another cohort study reported that only exceptionally severe psoriasis was associated with increased all-cause mortality.
The lack of overall consensus among current studies may be related in part to study size and an associated lack of power, as well as the different patient populations involved.
Lipoproteins and Risk of Cardiovascular Disease
Changes in lipoprotein metabolism and lipoprotein retention are key to initiation of an atherosclerotic lesion. Lowering low-density lipoprotein (LDL) levels has long been the primary target of therapy in most clinical guidelines, but it is increasingly recognized that the ratios between lipoproteins, particle size, and lipid function all play a role in reducing the risk of cardiovascular disease.
Alterations in LDL metabolism and particle size have been shown to promote atherogenesis. Other lipoprotein subfractions such as very low–density lipoproteins, intermediate-density lipoprotein, and chylomicron remnants are also proatherogenic; this finding has led to the understanding that non–high-density lipoprotein (HDL) cholesterol (remnant cholesterol—the cholesterol content of triglyceride-rich lipoproteins ) levels might be more strongly associated with the risk of a cardiovascular event than LDL cholesterol levels alone. Arsenault et al demonstrated that non-HDL cholesterol levels were correlated with risk of future coronary heart disease even in subjects with low LDL cholesterol levels. High concentrations of non-HDL cholesterol have also been associated with increased ischemic heart disease, independent of HDL. Of note, lipoprotein levels are not the only variables to consider, triglyceride-rich lipoproteins have been found to be independently involved in the recruitment of monocytes to inflamed arterial endothelium, thus driving the progression of atherosclerosis.
It has been proposed that smaller lipoprotein particles are more proatherogenic because they have a lower binding affinity for the LDL receptor, which leads to impaired clearance and increased circulation time.
Low levels of HDL are also associated with an increased risk of cardiovascular disease. HDL particles promote reverse cholesterol transport by removing excess cholesterol from macrophages and tissues and delivering the cholesterol to the liver ; this process limits the cytotoxicity of cholesterol. More recent epidemiologic evidence confirmed that HDL cholesterol efflux capacity is inversely related to the risk of incident cardiovascular disease. However, studies of the cholesteryl ester transfer protein inhibitor, dalcetrapib, showed that this agent raised HDL cholesterol levels but did not reduce the risk of cardiovascular events. Similarly, a meta-analysis of HDL-raising therapies in randomized controlled trials concluded that no drug that raised HDL reduced the incidence of myocardial infarction, stroke, or coronary heart disease mortality.
Accumulating evidence indicates that other lipoprotein measurements could also predict the risk of cardiovascular events. Analysis of 1 clinical trial found that although, in the setting of potent statin therapy, HDL-cholesterol level was not predictive of residual risk of heart disease, HDL particle number was a potential marker of residual risk.
Lipoproteins and Risk of Cardiovascular Disease
Changes in lipoprotein metabolism and lipoprotein retention are key to initiation of an atherosclerotic lesion. Lowering low-density lipoprotein (LDL) levels has long been the primary target of therapy in most clinical guidelines, but it is increasingly recognized that the ratios between lipoproteins, particle size, and lipid function all play a role in reducing the risk of cardiovascular disease.
Alterations in LDL metabolism and particle size have been shown to promote atherogenesis. Other lipoprotein subfractions such as very low–density lipoproteins, intermediate-density lipoprotein, and chylomicron remnants are also proatherogenic; this finding has led to the understanding that non–high-density lipoprotein (HDL) cholesterol (remnant cholesterol—the cholesterol content of triglyceride-rich lipoproteins ) levels might be more strongly associated with the risk of a cardiovascular event than LDL cholesterol levels alone. Arsenault et al demonstrated that non-HDL cholesterol levels were correlated with risk of future coronary heart disease even in subjects with low LDL cholesterol levels. High concentrations of non-HDL cholesterol have also been associated with increased ischemic heart disease, independent of HDL. Of note, lipoprotein levels are not the only variables to consider, triglyceride-rich lipoproteins have been found to be independently involved in the recruitment of monocytes to inflamed arterial endothelium, thus driving the progression of atherosclerosis.
It has been proposed that smaller lipoprotein particles are more proatherogenic because they have a lower binding affinity for the LDL receptor, which leads to impaired clearance and increased circulation time.
Low levels of HDL are also associated with an increased risk of cardiovascular disease. HDL particles promote reverse cholesterol transport by removing excess cholesterol from macrophages and tissues and delivering the cholesterol to the liver ; this process limits the cytotoxicity of cholesterol. More recent epidemiologic evidence confirmed that HDL cholesterol efflux capacity is inversely related to the risk of incident cardiovascular disease. However, studies of the cholesteryl ester transfer protein inhibitor, dalcetrapib, showed that this agent raised HDL cholesterol levels but did not reduce the risk of cardiovascular events. Similarly, a meta-analysis of HDL-raising therapies in randomized controlled trials concluded that no drug that raised HDL reduced the incidence of myocardial infarction, stroke, or coronary heart disease mortality.
Accumulating evidence indicates that other lipoprotein measurements could also predict the risk of cardiovascular events. Analysis of 1 clinical trial found that although, in the setting of potent statin therapy, HDL-cholesterol level was not predictive of residual risk of heart disease, HDL particle number was a potential marker of residual risk.
Immunopathogenesis of Psoriasis
Psoriasis is considered to arise through complex signaling pathways that affect different constituents of the immune system ( Figure 2 ), each contributing to the pathogenesis of the disease. Briefly, interferon (IFN)-α released from plasmacytoid dendritic cells initiates the activation and maturation of myeloid dendritic cells. These cells migrate to draining lymph nodes, where they present antigens and release co-stimulatory signals and cytokines resulting in the differentiation of T cells. In particular, cytokines such as interleukin [IL]-6, IL-12, IL-23, and IFN-γ aid in the differentiation and expansion of T helper (Th) cells that often migrate back to epidermal and dermal tissues and interact with a variety of cells, including dermal and inflammatory dendritic cells, macrophages, mast cells, and neutrophils, to cause continued proliferation of keratinocytes as well as ongoing recruitment of T cells.
Levels of Th1-associated cytokines (tumor necrosis factor [TNF]-α, IFN-γ, and IL-2), and Th17-associated cytokines (IL-17A, IL-17F, IL-22, IL-26, and TNF-α), are elevated in the serum and lesional skin of patients with psoriasis. TNF and IL-17 are major drivers of the skin disease phenotype, as shown by disease improvements with multiple antagonists of these cytokines. IL-17, a cytokine generally involved in host defense against extracellular bacteria and fungi, plays a particularly important role. IL-17A is produced by Th17 lymphocytes, neutrophils, and mast cells. IL-17 acts on keratinocytes to increase the expression of chemokines involved in recruiting myeloid dendritic cells, Th17 lymphocytes, and neutrophils to the psoriasis lesion site. The key role of this cytokine is demonstrated by the dramatic clinical improvements reported in patients treated with the humanized anti–IL-17 monoclonal antibody, ixekizumab; patients experienced reversal of epidermal hyperplasia and dermal infiltration of leukocytes.
Inflammation and Endothelial Cell Dysfunction
Many processes have been implicated in early atherogenesis ( Figure 3 ), with endothelial dysfunction a crucial underlying mechanism for initiation and progression of cardiovascular diseases. The proinflammatory cytokine TNF-α has been associated with endothelial cell dysfunction alone and in combination with IL-17. It has been suggested that atherosclerotic plaque formation is dependent on inflammation and the production of IL-17, which is elevated at each stage of plaque development in atherosclerotic lesions. T cells have also been found to infiltrate arteries and coexpress IFN-γ and IL-17, producing a synergistic effect on cultured human vascular smooth muscle cells and leading to the secretion of proinflammatory cytokines (IL-6) and chemokines (CXCL8 and CXCL10), which are elevated in atherosclerosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


