Current guidelines recommend invasive coronary angiography and interventional revascularization in ST-elevation and Non-ST-elevation myocardial infarction (STEMI and NSTEMI). The aim of this study was to analyze culprit lesions and percutaneous coronary intervention (PCI) success in patients with previous coronary artery bypass grafting (CABG). We analyzed the data of 121 consecutive patients in whom coronary angiography was performed in the setting of STEMI or NSTEMI and who had previous CABG. Coronary angiograms were reviewed, and clinical data were evaluated. The culprit vessel was identified by means of electrocardiographic findings, echocardiography, and coronary angiography. A bypass graft was the culprit vessel in 86% of patients with STEMI and 68% of patients with NSTEMI. Venous grafts were culprit vessels significantly more frequently than arterial grafts (81 of 260 venous grafts vs 4 of 65 arterial grafts, p <0.001). Attempted acute PCI was successful in 97% of native arteries (31 of 32 patients) but only in 81% of bypass grafts (68 of 84 patients, p = 0.031). Overall in-hospital mortality was 13% (STEMI: 25%, NSTEMI: 10%, p <0.001) and was significantly higher after failed acute PCI (6 of 11 patients; 55%) compared with successful acute PCI (7 of 110 patients; 6%; p = 0.006). In conclusion, the culprit lesion in patients with previous CABG who present with STEMI or NSTEMI is more often located in bypass grafts than in native arteries. Successful PCI is more likely in native arteries compared with bypass grafts.
Current guidelines recommend invasive coronary angiography and interventional revascularization in acute myocardial infarction (NSTEMI and STEMI). About 10% to 15% of patients with acute myocardial infarction have previously undergone coronary artery bypass grafting (CABG). Hemodynamically relevant lesions both in bypass grafts and in native arteries are common after CABG and their prevalence increases with time. These lesions are major determinants of clinical events and survival. Identification of the culprit vessel and revascularization decisions can be complex in subjects with acute coronary syndromes and previous CABG. Nevertheless, identification and, if possible, revascularization of the culprit vessel within a narrow time frame is crucial to reduce mortality and improve clinical outcome. The intention for this study was to evaluate coronary angiographic data in patients who presented with STEMI or NSTEMI and had previously undergone CABG to characterize the distribution of culprit lesion location as well as revascularization success.
Methods
We retrospectively analyzed the data of all consecutive patients with previous CABG in whom coronary angiography was performed in the setting of STEMI or NSTEMI from January 2010 to May 2015. Data regarding clinical presentation, electrocardiography, and echocardiography were evaluated. STEMI was defined as acute myocardial ischemia with troponin I levels above 2.5 ng/ml (5 × the upper limit of normal) and significant ST-segment elevation (>0.2 mV in 2 adjacent chest leads and/or >0.1 mV in 2 adjacent limb leads) or new left bundle branch block. NSTEMI was defined as acute myocardial ischemia with troponin I above 2.5 ng/ml in the absence of significant ST-segment elevation. Coronary angiograms and all clinical data were reviewed in consensus by 2 experienced interventional cardiologists. The presence and distribution of ST-segment alteration in electrocardiogram as well as regional wall motion abnormalities (hypokinesia, akinesia, or dyskinesia in transthoracic echocardiography and/or in laevocardiography) were analyzed to identify the infarct territory, and, in conjunction with the angiogram, the culprit lesion. Successful percutaneous coronary intervention (PCI) was defined as angiographic restoration of TIMI 3 flow. Patients were excluded from analysis if the culprit vessel could not be unambiguously identified.
Continuous variables are summarized as mean ± SD; categorical variables are quoted as n (%). Kolmogorov–Smirnov test was performed to test for parametric distribution. To test for statistical differences between 2 groups for comparison of continuous variables either t test for unpaired samples (parametric distribution) or Mann–Whitney U test (nonparametric distribution) was performed. For categorical variables, chi-square or the Fisher’s exact test was carried out to test for significant differences. Statistical analysis was performed using SPSS, version 21.0 (IBM SPSS Statistics; IBM Corporation, Armonk, New York), and a 2-sided p <0.05 was considered significant.
Results
In 12 patients, the culprit vessel could not be unambiguously identified, and they were excluded, so that 121 cases remained for analysis. For detailed patient characteristics and clinical presentation, see Table 1 .
| Variable | |
|---|---|
| Age (years) | 74 (56; 84) |
| Male/Female | 98/23 |
| Body-Mass-Index (kg/m 2 ) | 27.6 (24.4; 36.8) |
| Clinical presentation | |
| Typical angina pectoris | 78 (65%) |
| Atypical chest pain | 7 (6%) |
| Symptoms other than chest pain | 36 (30%) |
| Glomerular filtration rate ≥ 60 mL/min | 104 (86%) |
| Left ventricular ejection fraction (%) | 48 (32; 60) |
| Left ventricular ejection fraction <30% | 24 (20%) |
| ST-segment elevation myocardial infarction/Non-ST-segment elevation myocardial infarction | 28 (23%) / 93 (77%) |
| Death | 16 (13%) |
| Time since last coronary artery bypass grafting (years) | 12 (1; 19) |
| Venous grafts per patient | 2.1 (1.4; 3.9) |
| Arterial grafts per patient | 0.5 (0.3; 2.1) |
| Time until discharge (days) | 3.4 (1; 8) |
| Infarct territory | |
| Left anterior descending | 46 (38%) |
| Circumflex artery | 44 (36%) |
| Right coronary artery | 31 (26%) |
| Lesion type (ACC/AHA-classification) | |
| A | 4 (3%) |
| B1 | 12 (10%) |
| B2 | 84 (69%) |
| C | 21 (17%) |
Patients with a culprit lesion located in a bypass graft more frequently presented with STEMI compared to patients with a culprit lesion in a native coronary artery. However, this difference was not significant (28% vs 11%; p = 0.17). A bypass graft was the culprit vessel in 86% of patients with STEMI (24 of 28) and 68% of patients with NSTEMI (63 of 93; p = 0.064). If the culprit lesion was within a native coronary artery, it was distal to a bypass anastomosis in 6 of 34 cases (18%) and in a nongrafted artery in 28 of 34 cases (82%; p <0.001). Venous grafts were culprit vessels significantly more frequently than arterial grafts (81 of 260 venous grafts vs 4 of 65 arterial grafts, p <0.001). The likelihood of a bypass graft being the culprit vessel showed a trend toward increase with the total number of venous grafts (Spearman’s rank correlation coefficient: 0.181; p = 0.054) a patient had received but not with the number of arterial grafts (Spearman’s rank correlation coefficient: −0.06; p = 0.645). See also Tables 2 and 3 .
| Number of Venous Bypass Grafts | Probability for Any Bypass Culprit Lesion | Number of Patients |
|---|---|---|
| 0 | 17% | 6 |
| 1 | 58% | 19 |
| 2 | 73% | 51 |
| 3 | 81% | 38 |
| 4 | 100% | 7 |
| Number of Arterial Bypass Grafts | Probability for Any Bypass Culprit Lesion | Number of Patients |
|---|---|---|
| 0 | 81% | 57 |
| 1 | 67% | 61 |
| 2 | 0% | 2 |
| 3 | 0% | 1 |
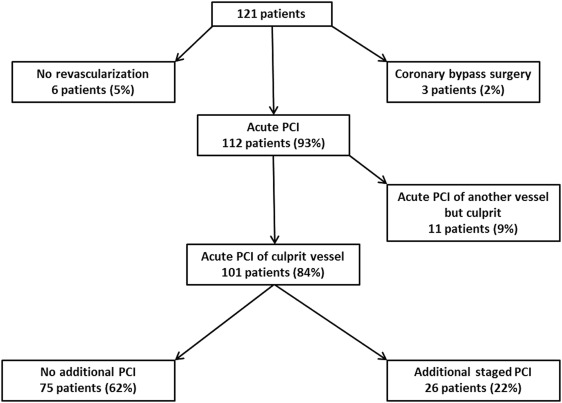
Acute PCI of the culprit vessel was performed in 101 patients (84%). If the culprit vessel was a bypass graft, acute PCI of the bypass was performed in 69 of 87 cases (79%), whereas in 15 of 87 cases (16%), acute PCI of the native artery once supplied by the graft was performed. This was due to graft occlusion in 9 of 15 cases and due to ectatic or tortuous graft anatomy in 6 of 15 cases. If the culprit vessel was a native artery, acute PCI was performed in 32 of 34 cases (94%). In 21 patients (17%), PCI of the culprit vessel and another artery was performed simultaneously, whereas in 11 patients (9%), the culprit vessel was not revascularized, but PCI of another territory was performed in the initial procedure. In one case (1%) emergent bypass surgery and in 2 cases (2%) deferred bypass surgery were recommended. Additional staged PCI was performed in 26 patients (21%). No revascularization was performed in 6 cases (5%). See also Figure 1 .
Attempted acute percutaneous revascularization was successful in 81% of bypass grafts (68 of 84 patients) and 97% of native arteries (31 of 32 patients; p = 0.031).
In-hospital mortality was 13% (STEMI: 25%, NSTEMI: 10%, p <0.001). Of 32 patients with a culprit lesion located in a native artery, 3 patients (9%) died before discharge after initially successful PCI. Of 89 patients with a culprit lesion located in a bypass graft, 13 patients (15%) died before discharge (p = 0.43). In-hospital mortality was 7 of 110 patients (6%) after successful acute PCI versus 6 of 11 patients (55%) after failed acute PCI (p = 0.006). For detailed procedural and outcome data see also Table 4 .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


