, Jorge Plutzky2 and Jorge Plutzky3
(1)
Harvard Medical School Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
(2)
Harvard Medical School, Boston, USA
(3)
Vascular Disease Prevention Program, Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA
Abstract
Disorders of serum lipid and lipoproteins account for approximately 50 % of population-attributable risk for myocardial infarction (MI) [1, 2]. Randomized control trial (RCT) data demonstrate that treating elevated levels of low density lipoprotein (LDL) cholesterol results in substantial reductions in cardiovascular events and death [3]. Evidence-based guidelines identify serum lipids and lipoprotein disorders as important targets in primary and secondary prevention of coronary heart disease (CHD) [4, 5].
Abbreviations
ABCA
ATP-binding cassette transporter A
ABCG
ATP-binding cassette transporter G
ACS
Acute Coronary Syndrome
ATPIII
Adult Treatment Panel III
CAD
Coronary artery disease
CE
Cholesterol esters
CETP
Cholesterol ester transfer protein
CHD
Coronary Heart Disease
CK
Creatine kinase
CKD
Chronic kidney disease
CM
Chylomicron
CV
Cardiovascular
DM
Diabetes Mellitus
FA
Fatty acid
FC
Free cholesterol
FCH
Familial combined hyperlipidemia
FDB
Familial defective apolipoprotein B
FFA
Free fatty acids
FH
Familial hypercholesterolemia
FRS
Framingham Risk Score
GFR
Glomerular Filtration Rate
GI
Gastrointestinal
HDL
High density lipoprotein
HDL-C
High density lipoprotein cholesterol
IDL
Intermediate density lipoprotein
LCAT
Lecithin cholesterolacyl transferase
LDL
Low density lipoprotein
LDL-C
Low density lipoprotein cholesterol
LDL-R
LDL receptor
LFTs
Liver function tests
LPL
Lipoprotein lipase
MetS
Metabolic syndrome
MI
Myocardial Infarction
Non-HDL-C
Non high density lipoprotein cholesterol
NCEP
National Cholesterol Education Program
PCI
Percutaneous intervention
PL
Phospholipids
PPARα
Peroxisome proliferator-activated receptor alpha
RCT
Randomized control trial
RF
Risk factors
TC
Total cholesterol
TLC
Therapeutic lifestyle changes
TG
Triglycerides
T2D
Type 2 diabetes mellitus
VLDL
Very low density lipoprotein
Introduction
Disorders of serum lipid and lipoproteins account for approximately 50 % of population-attributable risk for myocardial infarction (MI) [1, 2]. Randomized control trial (RCT) data demonstrate that treating elevated levels of low density lipoprotein (LDL) cholesterol results in substantial reductions in cardiovascular events and death [3]. Evidence-based guidelines identify serum lipids and lipoprotein disorders as important targets in primary and secondary prevention of coronary heart disease (CHD) [4, 5].
Overview of Lipoprotein Metabolism
Three major types of lipids circulate in the serum:
Cholesterol-essential component of cell membranes and substrate for synthesis of steroid hormones and bile acids
Triglycerides (TG)-macromolecules consisting of glycerol backbone connected to three fatty acids (FA)
Phospholipids (PL)-important constituents of cell membranes
Serum lipids are packaged and transported in lipoproteins (Fig. 6-1), which consist of a hydrophobic lipid core of TG, and a polar shell consisting of hydrophilic PL, free cholesterol (FC), and specialized apolipoproteins.
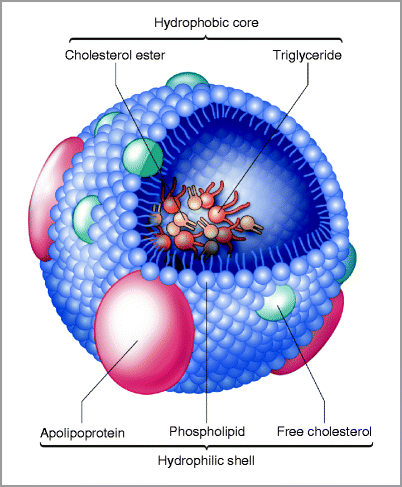
Figure 6-1
Schematic of a lipoprotein
Lipoproteins are classified according to their density in plasma (Table 6-1)
Table 6-1
Plasma lipoproteins
Lipoprotein
Density (g/mL)
Primary lipid component
Major apolipoprotein
Chylomicron (CM)
<0.95
TG
B48
CM remnant
0.95–1.006
TG
B48, E
Very low density lipoprotein (VLDL)
<1.006
TG
B100
Intermediate density lipoprotein (IDL)
1.006–1.019
TG, CE
B100, E
LDL
1.019–1.063
CE
B100
High density lipoprotein (HDL)
1.063–1.210
CE, TG
A-I, A-II
Lipoprotein(a) or Lp(a) is an LDL-like lipoprotein that confers a modest increased risk of CHD independent of LDL-C
Lp(a) contains the Apo(a) apolipoprotein, which has structural homology to plasminogen but lacks any fibrinolytic activity
Apo(a) may interfere with plasminogen activity and contribute to the pro-thrombotic state associated with Lp(a)
Routine measurement of Lp(a) is not recommended as a part of risk factor assessment
Apolipoproteins (Apo; Table 6-2) participate in:
Table 6-2
Major apolipoproteins
Apolipoprotein
Predominant lipoprotein
Role
A (A-I, A-II, A-IV, A-V)
HDL
ACAT activation (A-I)
VLDL (A-V)
Structural integrity (A-II, A-IV)
TG metabolism (A-V)
B48
CM, CM remnants
Structural integrity
B100
VLDL, IDL, LDL
Structural integrity
LDL-R binding
C (C-I, C-II, C-III)
CM (C-I, C-II, C-III)
TG Metabolism (C-I)
VLDL (C-II, C-III)
LPL activation (C-II)
LPL Inhibition (C-III)
E
CM remnants, IDL
LDL-R, Apo E-Receptor binding
lipoprotein assembly and secretion (Apo A-I, Apo B100, Apo B48)
catalysis (Apo A-I, Apo A-V, Apo C-II) or inhibition (Apo C-III) of enzymes
lipoprotein binding to receptors (Apo B48, B100)
Lipoprotein transport serves two important functions:
Transport of TG from the intestine to the liver and sites of TG uptake (muscle & fat)
Transport of cholesterol to peripheral tissues
Two important pathways coordinate lipoprotein transport:
Intestinal pathway (Fig. 6-2 ):
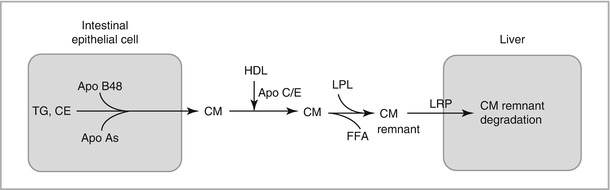
Figure 6-2
Schematic of intestinal pathway. Chylomicrons (CM) are assembled in intestinal epithelial cells and secreted into the circulation, where they undergo lipolysis by lipoprotein lipase (LPL). CM-remnants are taken up by the liver. LRP lipoprotein receptor-related protein
Coordinates transfer of dietary TG to the liver and peripheral tissues
Dietary TG in intestinal epithelial cells are packaged with Apo B48 and a small amount of cholesterol to form chylomicron (CM) lipoproteins
CM are secreted into the circulation and acquire Apo C and E from HDL
CM undergo hydrolysis by lipoprotein lipase (LPL) on endothelial cells
Free fatty acids (FFA) released by TG hydrolysis are taken up for energy storage
Hepatic Pathway (Fig. 6-3 ):
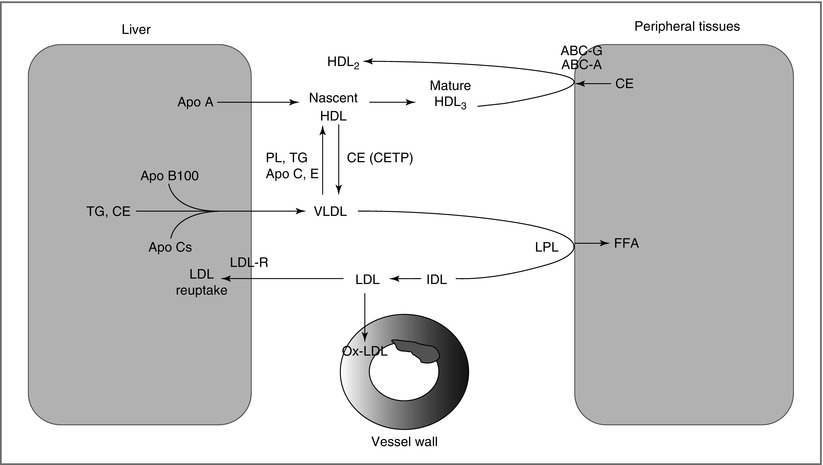
Figure 6-3
Schematic of hepatic pathway. TG-rich VLDL are assembled in the liver and secreted into the circulation. Cholesteryl ester transfer protein (CETP) facilitates transfer of TG from VLDL to HDL in exchange for cholesterol. VLDL is hydrolyzed by LPL to release FFA that are taken up by peripheral tissues (fat, muscle). LDL generated from LPL action can enter the vessel wall, where its oxidation and uptake by macrophages leads to foam cell formation, a key step in atherogenesis. Nascent HDL secreted from the liver play an important role in reverse cholesterol transport
Coordinates the transport of TG and cholesterol between the liver and target peripheral tissues
Hepatocytes package TG with Apo B100 to form VLDL
In the circulation, VLDL interacts with HDL to [1]: acquire additional lipoproteins (Apo C and E), and [2] exchange TG for CE
Like CM, VLDL undergoes lipolysis by LPL to produce FFA
VLDL hydrolysis yields IDL, which undergoes further hydrolysis by hepatic lipase to form LDL, the main courier of FC and CE
LDL is taken by the liver, a process mediated by the LDL receptor (LDL-R)
LDL modification in the vessel wall and uptake by macrophages leads to foam cell formation, a key step in atherogenesis
Lipoprotein Disorders and CHD Risk
Several lipid and lipoprotein variables have been reported to confer an increased risk of CHD:
A continuous log-linear relationship exists between LDL-C and CHD risk [5]:
a decrease of 1 mg/dL in LDL-C results in a 1 % decrease in CHD relative risk
a given mg/dL change in LDL-C produces the same change in relative risk of CHD at any level of LDL-C
Although LDL-C is the primary lipid target, other parameters such as low HDL-C, non-HDL-C, and Apo B appear to be stronger predictors of CHD risk [9, 12, 13]
An increase of 1 mg/dL in HDL-C is associated with a 2–3 % decrease in CHD risk [8], but to date there is no RCT to support targeting HDL-C
ATPIII identified non-HDL-C, a surrogate marker of Apo B levels, as a secondary target in patients with TG > 200 mg/dL (Non-HDL-C = TC – HDL-C)
NCEP ATPIII guidelines did not recommend routine testing for emerging lipid risk markers
Diagnosis and Screening
The most common lipoprotein disorders encountered clinically are related to age, physical inactivity, diet, obesity, and lifestyle factors (smoking)
Recent classification systems from WHO and NCEP focus on thresholds and cut points for serum lipoprotein lipids to diagnose and treat lipoprotein disorders (Table 6-3)
Table 6-3
Serum lipid concentrations
Lipid
Optimal (mg/dL)
Borderline elevation (mg/dL)
Elevated/high risk (mg/dL)
TC
<200
200–239
>240
LDL-C
<100 (100–129-near optimal)
130–159
>160
HDL-C
≥60
40–59 (men)
<40 (men)
50–59 (women)
<50 (women)
TG
<150
150–199
>200
Plasma levels of lipids (cholesterol and triglycerides) and lipoprotein cholesterol (LDL-C and HDL-C) define three general patterns of lipoprotein disorders commonly encountered in clinical settings:
Hyperlipidemia refers to an elevation in LDL-C (and TC)
Hypertriglyceridemia refers to an elevation in TG
Dyslipidemia refers to a combination of hypertriglyceridemia and low HDL-C with either a normal or elevated TC or LDL-C (commonly seen in insulin resistant states)
Fasting lipid profile (FLP), which provides direct measurement of TC, TG, HDL-C and calculated LDL-C, is the preferred initial test, rather than measurement of non-fasting TC and HDL-C alone.
The Friedewald formula [14] (below) is used to calculate LDL-C, because direct measurement of LDL-C is time consuming and costly
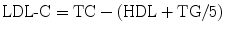
The LDL-C calculated from the Friedewald formula is inaccurate at higher TG levels [15], and direct measurement of LDL should be considered if TG > 400 mg/dL [4, 15]
ATP III screening guidelines: FLP at least once every 5 years for individuals older than 20 years of age, with consideration for more frequent testing in older individuals with risk factors [4]
Significant elevations in LDL-C (> 190 mg/dL) can indicate a genetic disorder that warrants further consideration and family testing
Patients admitted with MI should have a FLP within 24 h of admission (levels drawn later will be spuriously low due to hepatic stress response or medications)
Management of Lipoprotein Disorders
Drugs that Modulate Lipid Metabolism
Hyroxymethylglutaryl-Coenzyme A Reductase Inhibitors (Statins)
Statins inhibit HMG-CoA reductase, the rate-limiting enzyme in sterol synthesis
Statins increase expression of LDL-R and decrease CE formation, leading to enhanced LDL-C clearance from plasma and reduced VLDL production
Statins are the drugs of choice for reducing LDL-C (20–55 % reduction)
Statins also modestly decrease TG (5–30 %) and increase HDL-C (2–10 %)
Data support efficacy of statins in primary and secondary prevention across age groups, in men and women, and in type 2 diabetes (T2D) [3, 4]
Starting statin dose should be sufficient to decrease the LDL-C by 30–40 % [4] (Table 6-4)
Table 6-4
Doses of available statins required to attain 30–40 % reduction in LDL-C
Statin
Dose (mg/dL)
LDL-C reduction (%)
Fluvastatin< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
