Fig. 35.1
Totally aortic “non-touch” technique in off-pump three-vessel coronary artery bypass grafting surgery via left minithoracotomy; the inflow vein grafts come from the distal left subclavian artery in addition to in situ left internal mammary artery graft
35.5 Technological Innovations
New technologies have played a crucial role in the evolution of less invasive cardiac surgery. Importantly, they have changed the perceptions of cardiac surgeons regarding how cardiac surgery can or should be performed. With the help of new instruments specifically designed to meet the surgeon’s need, less invasive cardiac surgical procedures once deemed impossible or impractical have now become reality, or even common practice, in some medical centers. These technological innovations have typically involved the following aspects of cardiac surgery.
35.5.1 Sternum-Sparing Surgery: Partial Sternotomy, Minithoracotomy, and Thoracoscopy
Major advances in this area include the development of a cardiopulmonary bypass support system via peripheral access. The application of suction to the venous drainage has made possible aortic valve and mitral valve surgery via partial sternotomy and minithoracotomy. An earlier breakthrough device in this field was the HeartPort system (developed by Stanford University and New York University Hospital in 1994) which was composed of peripheral vessel-based cardiopulmonary bypass perfusion, an endo aortic balloon occlusion catheter, transvenously placed venting and cardioplegia cannulas, and extra-long operating instruments. Though its early use proved impractical in most cardiac operations, its potential to be less invasive has significantly changed cardiac surgeons’ and medical engineers’ perception of future technologies. Furthermore, the concept of the HeartPort system led to numerous other technological modifications and innovations in the field of less invasive cardiac surgery. Such innovations include: (1) the development of small caliber multistage peripheral venous cannula, (2) the safe application of vacuum-assisted venous drainage to ensure bloodless exposure inside heart, (3) the small thin blade minithoracotomy retractor and atrial retractor, (4) development of the Chitwood aortic cross-clamp, (5) thoracoscopy or endoscopic robotics to assist in the mitral valve repair or replacement, and (6) liberal use of transesophageal echocardiography to guide the insertion of various intracardiac cannulas.
35.5.1.1 Upper Partial Sternotomy or Minithoracotomy Approaches for Aortic Valve Replacement
Currently more and more aortic valve replacements are performed via upper partial sternotomy or minithoracotomy. In such procedures, a limited partial sternotomy is made and the splitting of sternum is terminated at the 3rd or 4th intercostal space with either a “J” or inverted “T” incision, or a 6 cm incision is made at the right 2nd intercostal space (Fig. 35.2). Even an aortic valve replacement surgery can be performed via such a small incision, by using a combination of central and peripheral cardiopulmonary bypass circuits. Note that the aortic valve can be adequately exposed and replaced (Figs. 35.3 and 35.4).
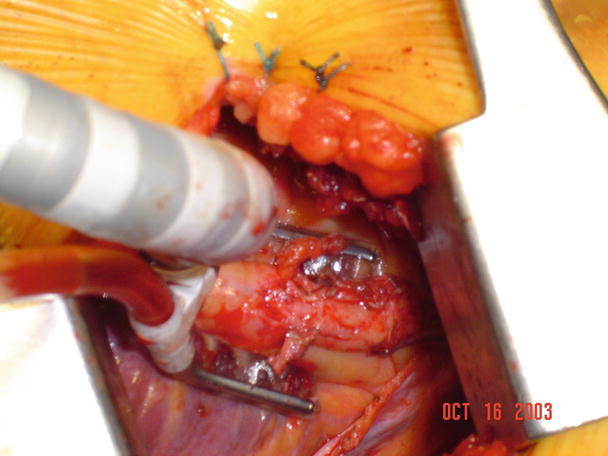
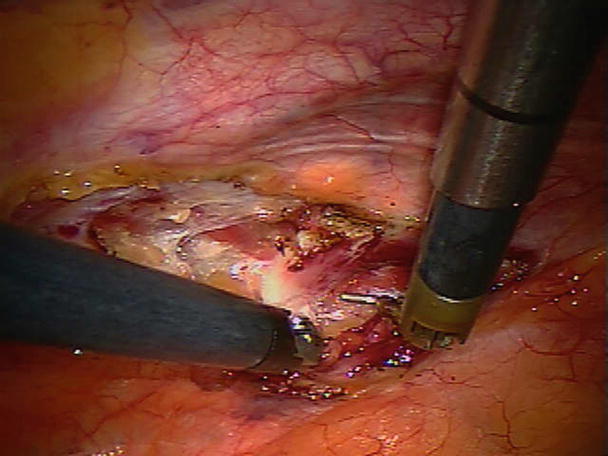


Fig. 35.2
Drawing of incisions used in minimally invasive aortic valve replacement. An upper sternotomy incision ends at the 3rd intercostal space at a “J” angle; a minithoracotomy incision is located at the right 2nd intercostal space

Fig. 35.3
The small incision allows for insertion of a combination of central and peripheral cannula for the establishment of cardiopulmonary bypass

Fig. 35.4
The exposure is adequate for aortic valve replacement. A bioprosthetic valve is visible
A right minithoracotomy procedure is increasingly being used for minimally invasive mitral valve repair and/or replacement surgery. In these procedures, a 6 cm incision is typically made at the right 3rd intercostal space and a specially designed small retractor is inserted. A combination of central and peripheral cardiopulmonary bypass circuits is established, and intracardiac cannulas are inserted under the guidance of transesophageal echocardiography (Fig. 35.5). Currently, special instruments including clamps, scissors, forceps, and a knot-tying device are used for these procedures (Fig. 35.6). In other words, both mitral valve repair and replacement procedures can be safely performed using this approach (Fig. 35.7). The main advantages of these procedures are decreased blood loss and the quick return of the patient to physical activities when compared to conventional sternotomy approach (Fig. 35.8).
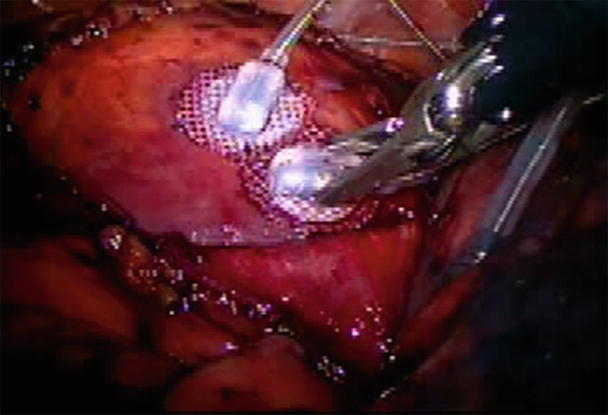
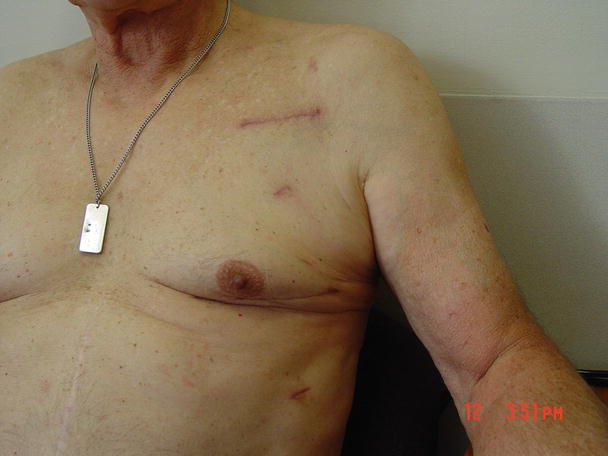
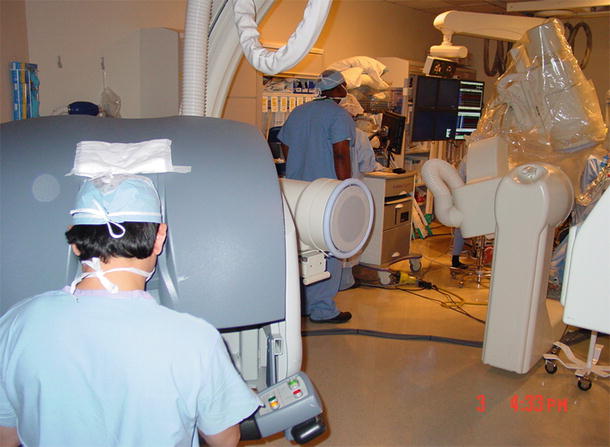

Fig. 35.5
A right minithoracotomy incision at the 3rd intercostal space. A combination of central and peripheral cannula insertion is used for the establishment of cardiopulmonary bypass

Fig. 35.6
Special instruments with extra-long handles and small tips are used for minimally invasive mitral valve surgery

Fig. 35.7
Mitral valve repair with minithoracotomy can achieve good exposure

Fig. 35.8
Minithoracotomy results in less blood loss, quick return to work, and a better cosmetic outcome
35.5.2 OPCABG Improvement
New instruments have also been developed to position the heart and to stabilize and improve the visualization of target arteries. For example, an available left ventricle suction device applies −400 mmHg suction to the left ventricular apex and can hold the heart up in different positions. Now widely used in OPCABG surgery, this device has less of an effect on the venous return as compared with the old “suture retraction” technique. Similarly, a focal myocardial stabilization device has been developed to stabilize segments of target arteries; it has both suction and compressing effects on the topical epicardial tissue and thus significantly decreases the motion of target arteries (Fig. 35.9). An additional noteworthy device is the temporary intracoronary plastic shunt that can be inserted via arteriotomy to maintain blood flow to the distal myocardium during anastomosis, thus avoiding or minimizing ischemia time. Importantly, the use of such a shunt is considered to be crucial when the target artery supplies a large territory of myocardium. In order to facilitate the distal coronary anastomosis during OPCABG, especially in the anatomically difficult-to-reach areas, two innovative distal coronary artery anastomotic devices, C-PortxA (for the open sternotomy approach) and C-Port Flex A™ (for the minithoracotomy or endoscopic robotic approach) (Cardica Inc., Redwood City, CA, USA), were developed and recently approved for clinical use by the FDA. It should be noted that although the early clinical results of such devices are encouraging [22], their clinical adaption has been lackluster.
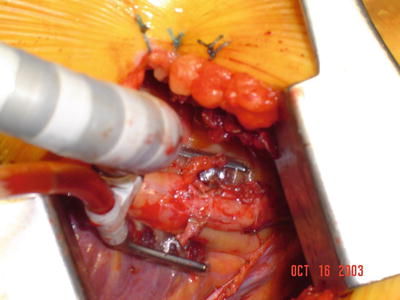

Fig. 35.9
An “octopus” myocardium-stabilizing device was used to steady the coronary artery during direct bypass grafting anastomosis
35.5.3 Aortic Non-touch Techniques
Different proximal anastomotic devices or hand-sewn facilitators have been developed and used to avoid clamping on the aorta during OPCABG surgery. Unfortunately, the clinical performance of most of these devices has been unsatisfactory, resulting in denial of FDA approval or termination of the products after FDA approval, i.e., the previously FDA-approved symmetry (St. Jude Medical, St. Paul, MN, USA) automated proximal connector being one example.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


