Lesions Masquerading as Primary Mediastinal Tumors or Cysts
Thomas W. Shields
The most common cause of a mediastinal enlargement is metastatic disease in the lymph nodes of the visceral and, at times, anterior compartments of the mediastinum from primary carcinoma of the lung. Occasionally, metastatic disease from other sites may cause a similar mediastinal enlargement, but by and large the diagnosis in the vast majority of such patients poses no major clinical problem. However, a lesion presenting as a mediastinal mass but actually arising originally from without the borders of the mediastinum or from one of the viscera within the mediastinum may also masquerade in its clinical or radiographic presentation as a primary mediastinal tumor or cyst. The more common, so-called masquerading lesions arise from the cervical region (substernal thyroid goiter and infrequently cystic hygroma), from the great vessels and heart (see Chapter 176), from below the diaphragm (hiatal hernias, foramen of Morgagni hernias, and pancreatic pseudocysts), from lesions of the other organs of the thorax (esophagus and lung), and less commonly from the thoracic skeleton and spinal canal (thoracic paravertebral abscess, thoracic chordoma, and anterior meningocele). Rarely, ectopic extramedullary hematopoietic tissue may be identified in the paravertebral sulci or even in the anterior compartment.
Intrathoracic Goiter
Definition
Wakeley and Mulvany,120 in their seminal report, divided intrathoracic goiters into three types: (a) “small substernal extension” of a mainly cervical thyroid goiter; (b) “partial” intrathoracic goiter, in which the major portion of the goiter is situated within the thorax; and (c) “complete” intrathoracic goiter, in which all of the goiter lies within the thoracic cavity. Of all the intrathoracic goiters, the small substernal extension is by far the most common and accounts for over 80% of goiters in most of the reported series. In Wakeley and Mulvany’s120 series, with an overall incidence of 8.7% for substernal goiters, the incidences of the three types were 81.9%, 15.3%, and 2.7%, respectively. The last two categories actually comprise only 1.6% of all goiters.
Incidence
Intrathoracic goiters are encountered less often today than they were in previous decades. Nonetheless they still must be considered as a diagnostic possibility in all anterosuperior mediastinal masses, particularly in eastern Europe and all of Asia. Katlic and associates44 reported 80 cases from the Massachusetts General Hospital from 1976 through 1982, and Dahan and associates21 reported 292 cases from the University Hospital Purpan, Toulouse, France, between 1977 and 1986.
De Andrade22 reported a total of 1,300 intrathoracic goiters in a series of 9,100 patients with goiters, an incidence of 14.2%. Of these intrathoracic goiters, 128 were defined as being partially or completely within the thorax, an incidence of 9.8% (or an incidence of 1.4% of all patients with goiters). This compares remarkably well with the incidence of 1.6% of partial or complete intrathoracic goiters reported by Wakeley and Mulvany in 1940.120 McCort59 reported a somewhat higher incidence of 3.1%, and Creswell and Wells17 reported that thyroid masses accounted for 5.8% of all mediastinal masses.
Anatomic Features
The vast majority of thoracic goiters with only a small substernal extension are located anteriorly in the visceral compartment and lie on the undersurface of the manubrium of the sternum on the cephalad aspect of the great vessels. These vessels may be displaced caudally and even somewhat dorsally. The impression may be gained that the goiter has descended slightly into the prevascular (anterior) mediastinal compartment, as suggested by Sweet111 and reiterated by Dahan and colleagues21 as well as by Newman and Shaha.66 Sweet and coworkers111 stated that 75% of their substernal goiters were prevascular and only 25% retrovascular. Dahan and colleagues21 stated that the goiters were located in the anterior mediastinum in 75% to 90% of cases and in the posterior mediastinum in 10% to 25% of cases. However, the anterior substernal extension actually remains in the visceral compartment because it is confined anteriorly by the pretracheal fascia. The goiter is thus prevented from entering the prevascular space that lies between this layer and the more superficial investing layer of the deep cervical fascia. However,
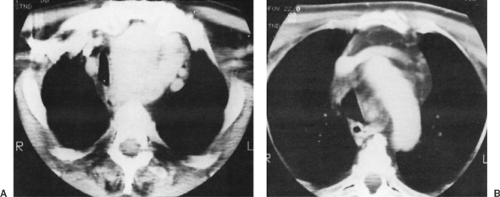
the goiter is located behind and medial to the great vessels as it descends into the thorax in close relationship to the trachea. McCort59 initially made this observation and rightly stated that the vessels in the superior portion of the mediastinum were the border-forming structures of the substernal thyroid goiters. His observation has been amply documented by the computed tomography (CT) characteristics of these lesions in the studies of Glazer34 and Bashist3 and their associates as well as by others, including my own observation96 (Fig. 175-1).
the goiter is located behind and medial to the great vessels as it descends into the thorax in close relationship to the trachea. McCort59 initially made this observation and rightly stated that the vessels in the superior portion of the mediastinum were the border-forming structures of the substernal thyroid goiters. His observation has been amply documented by the computed tomography (CT) characteristics of these lesions in the studies of Glazer34 and Bashist3 and their associates as well as by others, including my own observation96 (Fig. 175-1).
The vast majority of the true partial and completely intrathoracic thyroid lesions remain anterior or lateral to the trachea within the visceral compartment. In McCort’s59 series of 28 patients in this category, 19 (68%) of the thyroid masses were in this location, whereas 6 were retrotracheal, and 3 were located posterior to the esophagus, a total of 12% thus being retrotracheal in location. In the literature, these last lesions are often referred to as posterior goiters. Dahan and associates21 reported that 86% of their 75 posterior substernal goiters were retrotracheal, almost always on the right side; 4% were retroesophageal; 4%, although arising from the left lobe, were anterior and to the right of the trachea; and 6% were circumferential, or ring-shaped, about the trachea. The greater number of partial or complete intrathoracic goiters, even many of those arising from the left lobe, are located on the right. This most likely results from the position of the arch of the aorta on the left. Most of the lesions are confined to the area above the level of the aortic arch and, regardless of the lobe of origin, the lesion in most cases is confined within the basket formed by the great vessels anterior to the trachea. Further descent, even to the level of the diaphragm, has occasionally been observed.
Actual descent of a partial or complete intrathoracic goiter from the neck into the prevascular space occurs but is less common than descent into the postvascular space of the mediastinum. Many of the true examples of extension into the prevascular space have been recorded in patients with previous thyroid surgery in whom the fascial planes sealing this compartment undoubtedly have been violated by the previous surgical procedure. Ellis and associates28 reported that all nine patients with goiters in this location had had previous thyroid operations. Examples of a complete substernal thyroid mass in the prevascular compartment demonstrated by CT have been published by both Glazer34 and Bashist3 and their associates. In Bashist’s report, the patient had a previous goiter removed. Of course, partial or complete intrathoracic goiters that originally enter the mediastinum via the visceral compartment and are anterior or partially anterolateral to the trachea may, once within the mediastinum, pass in front of the ascending arch of the aorta on the right. Thus their lowermost portion may come to lie in the anterior compartment (the prevascular space) below the innominate vessels at the inlet. An invasive malignant tumor of the thyroid, either a primary or recurrent lesion, may also invade into the anterior compartment.
The blood supply of almost all partial and complete intrathoracic goiters arises from the inferior thyroid vessels. In a few patients with the complete variety, especially those with goiters that descend beyond the levels of the midthorax, and in those who have had previous thyroid surgery, a neovascular supply and drainage may be from or to one or more of the primary vessels within the thorax. However, this variance in blood supply does not in itself indicate that the site of origin of the tissue was not from the original thyroid tissue in the neck.
Almost all examples of so-called heterotopic (aberrant or ectopic) thyroid goiters described in the literature—such as most of the cases presented by Le Roux,50 Dundas,26 and Nwafo68 and probably those of Hall and associates39—represent intrathoracic goiters derived from a lower pole of a goitrous gland in the neck, whether or not a direct connection or normal vascular supply to the displaced tissue was present. This is especially true of the goiters located in the visceral compartment adjacent to the trachea and includes the large goiters that have descended to the level of the diaphragm. Almost every patient with a so-called ectopic thyroid goiter has had a previous, simultaneous, or subsequent goiter removed from the cervical gland. The report of a malignant tumor in an ectopic mediastinal thyroid by Sand and colleagues88 probably also falls into this category, because a previous resection of a benign follicular goiter had been performed 8 years before the discovery of the malignant lesion in the mediastinum.
True Ectopic Thyroid Tissue
Rarely, a normal nongoitrous or nearly normal thyroid mass has been identified in the anterior compartment in the vicinity of the thymus gland. An anomalous blood supply from a major great vessel within the thorax is always present. In almost all cases reported, the mass has been associated with a normal-sized and functioning thyroid gland in the neck, as determined by thyroid scintigraphy. This feature is in contrast to the anomalous lingual thyroid at the base of the tongue, which frequently is the patient’s only functioning thyroid tissue.
Embryologically, the thyroid anlage arises from a midline diverticulum of the floor of the pharynx at a level between the first and second pharyngeal pouches. This site is identified subsequently as the foramen cecum of the tongue. The tissue develops into a bilobed structure that ends its descent at the level of the laryngeal primordium. In the adult, the lower poles of the gland usually reach the level of the first tracheal ring, although abnormal descent to the sixth ring has been recorded. The major ectopic locations of thyroid are located from the upper poles of the gland to the base of the tongue. Sackett and associates86 identified nongoitrous thyroid tissue rests arising from the lower poles of the gland along the thyrothymic tract in the lower cervical area caudad to the thyroid gland. Such rests had not been described previously. These researchers noted such thyroid rests in 53 of 100 patients undergoing either thyroid or parathyroid operations. Eighty-five rests were identified, most (80%) of which were either protrusions from an inferior pole of the gland or were attached to it by a narrow pedicle of thyroid tissue. Another 9% of the rests were attached to a lower pole only by a fibrovascular band. Of more interest was that nine (11%) of the rests located in the thyrothymic tract were completely separate from the respective lower poles. Unfortunately, in no patient was the anterior mediastinum examined, so from these studies it remains unknown whether or not such rests occur associated with the thyrothymic tract within the mediastinal compartment. In contrast to the presence of ectopic parathyroid tissue in or adjacent to the thymus (see Chapter 164), I am aware of only rare reports of isolated islets of normal thyroid tissue either in or adjacent to the thymus, although three cases of a thyroid mass in the thymus have been reported, as noted below.
An illustration of an ectopic thyroid follicle located in the parathymic mediastinal fat was published by Meissner and Warren,60 but these investigators remarked that the presence of such tissue was of little clinical consequence. How common this occurrence is remains unknown. The report of extensive dissection of the anterior mediastinal area during thymectomy by Jaretzki and Wolff41 fails to note the identification of thyroid tissue located in the mediastinal fat removed during the operation. Gilmour,33 in an extensive study of the parathyroid glands in a large series of autopsy specimens, mentioned the occasional difficulty of gross identification of parathyroid tissue from accessory thyroid nodules but failed to describe the anatomic location of these accessory nodules. However, as noted by Meissner and Warren,60 such nodules are most commonly located adjacent to the normal thyroid gland in the neck. Thus it may be assumed that few if any were located in the mediastinum. From the data available, therefore, the presence of true ectopic thyroid tissue in the anterior compartment of the mediastinum is exceedingly uncommon. Likewise, no such tissue has been identified paratracheally within the thorax. It is possible that ectopic tissue from the bilateral postbronchial bodies from the rudimentary fifth pharyngeal pouches, which are believed normally to become incorporated and differentiated into normal thyroid, could be carried down into either the anterior or visceral compartments. According to Rogers,81 however, if these postbronchial bodies fail to be incorporated into the thyroid, differentiation into thyroid tissue does not occur, but such tissue may give rise to a small cystic structure adjacent to the trachea in the neck or the vascular compartment within the mediastinum.
Nonetheless, despite these observations, single examples of true ectopic thyroid masses in the mediastinum have been reported by numerous investigators. There have been insufficient criteria to establish the diagnosis of an ectopic thyroid mass in the anterior mediastinum. These few criteria have been that the mass be separated from the gland in the neck and that its blood supply be from vessels arising within the thorax rather than from the inferior thyroid vessel. To these, as the author has noted previously,98 the following additional criteria should be added: (a) the thyroid gland in the neck should be normal or completely absent; (b) no prior surgical removal of the “whole” or portion of the cervical gland should have been performed in the past; (c) no invasive malignancy of the thyroid gland should be present or should have been present in the past; and (d) no similar pathologic process should be present in either the cervical gland or the “ectopic thyroid tissue.” Only one of the several ectopic thyroid masses reported by Le Roux50 and those reported by Salvatore and Gallo86 and Sussman and associates108 were true ectopic thyroid lesions. I have had one such patient.98 In each instance normal thyroid tissue with or without involutional nodules was present in the anterior compartment in the vicinity of the thymus and disconnected from a normally functioning nongoitrous gland in the neck. The case reported by Houck and colleagues40 does not meet the aforementioned rigid criteria, but because of its aberrant blood supply, the lesion might well be ectopic in nature. However, the cases reported by Mishriki63 and Hall and colleagues39 do not meet the criteria outlined and probably should not be classified as ectopic lesions. On the other hand, the case reported by Grondin and associates36 meets all the appropriate criteria (Fig. 175-2). The true ectopic lesions should be treated as any other isolated anterior mediastinal mass. Surgical excision through a median sternotomy or by video-assisted thoracoscopic surgery (VATS) technique, as reported by Grondin and associates,36 is required.
Ectopic Thyroid Tissue in the Thymus
Spinner and colleagues105 first reported the presence of a thyroid tissue mass in the thymus gland in two men in 1994. There were no abnormalities of the cervical thyroid clinically, biochemically, or radiographically in either patient. The thyroid tissue mass was within the thymus in each patient and presented only as an anterior mediastinal mass. A third male patient reported by Tang and associates112 presented with a cervical goiter; during the preoperative evaluation, a left-sided anterior mediastinal mass was identified. Subsequently over 90% of the cervical thyroid goiter and the large mediastinal mass located at the lower pole of the left lobe of the thymus were removed. Histologically, the cervical gland was consistent with a typical thyroid goiter. The mediastinal mass within the lower pole of the thymus was also goitrous in nature; there was no evidence of any
connection to the cervical gland. This thyroid tissue most likely does represent true ectopic thyroid tissue located in the thymic gland.
connection to the cervical gland. This thyroid tissue most likely does represent true ectopic thyroid tissue located in the thymic gland.
Ectopic Thyroid Tissue in the Heart (Struma Cordis)
It is well recognized, as noted by Cove,16 that thyroid tissue can be displaced inferiorly during the embryogenesis of the heart as the result of a persistent contact between the thyroid anlagen to the bulbus cordis or from embryonic ectopic migration of these structures during the downward descent of neural crest cells to reach the outflow tract of the heart. As a consequence, thyroid tissue may be found in relation to the aortic arch, pericardium, and diaphragm as well as in the heart.
Although Dosch and associates24 were among the first to record the presence of thyroid tissue in the heart (interventricular septum and right ventricular outflow tract), Willis125 and Rogers and Kesten82 were the first investigators to identify and propose the embryologic basis for this occurrence. Rogers81 further discussed the possible explanations for the presence of ectopic thyroid tissue in the heart. Casanova and associates12 reviewed 20 case reports in the literature of intracardiac ectopic thyroid and added a case of their own. In addition, cases have been reported by Polvani73 and Baykut5 and their colleagues that were not included in the aforementioned study. Thus at least 23 examples of this rare condition have been recorded. Most of the patients were women (86.9%), most were in the fifth or sixth decade of life, and the intraventricular septum was involved as the major ectopic site in all patients. Nineteen patients had thyroid tissue obstructing the right ventricular outflow tract to a greater or lesser degree, two had involvement of the left outflow tract, and in two patients only the ventricular septum was involved and no outflow obstruction was noted. The symptoms were of outflow obstruction, ventricular arrhythmias, or both. Diagnosis is obtained by transthoracic echocardiography. Treatment is surgical removal of the intracardiac tissue under cardiopulmonary bypass.
Ectopic Thyroid Tissue in the Wall of the Ascending Aorta
Taylor114 and Williams124 and their associates each have described the rare occurrence of ectopic thyroid tissue present on the wall of the ascending aorta. The first was an autopsy observation and the second was discovered during a cardiac procedure. The thyroid tissue was easily dissected off the wall of the aorta. Postoperatively, studies revealed normal thyroid function. As noted, ectopic tissue associated with the heart and great vessels is most often seen in the interventricular septum at the right ventricular outflow tract, although at times the left outflow tract is involved.
Pathology
From the 1940s through the 1960s, most reported intrathoracic goiters were nontoxic multinodular goiters. Occasionally a toxic goiter was present. In Katlic and associates’44 report of 80 substernal thyroid lesions seen from 1976 through 1982, only 51% were multinodular goiters; 44% were follicular adenomas, and 5% were Hashimoto’s thyroiditis. Of the follicular adenomas, 23 were classified as simple, 4 as fetal, 2 as colloid, and 2 as embryonal; 3 were Hürthle cell lesions.
An occult papillary thyroid carcinoma may occasionally be found in the resected specimen of a substernal goiter. Wakeley and Mulvany120 and Katlic and associates44 reported an incidence of 2% to 3%. Dahan and colleagues21 reported an incidence of 5%. Rodriguez,80 Shai,94 and Makieff56 and their associates recorded incidences of 4%, 5.3%, and 1.4%, respectively. On the other hand, Allo and Thompson2 and Shaha and colleagues92 each recorded an incidence of thyroid carcinoma of 16%. How many cases in these two reports were clinically evident as opposed to occult in nature was not stated. Sanders and colleagues89 recorded an incidence of 21%. However, in this series, nine of the malignant lesions, including three cases of a lymphoma involving the thyroid, were clinically significant, and in only two patients (4.6%) was an occult tumor discovered
at the time of the excision of the substernal goiter. Torre and coworkers115 reported an incidence of 6.7% of cancer in their 237 cases of substernal goiter, but only 3 cases were of the occult type (1.2%). Of the other 13 patients with cancer, most had advanced, obvious clinical malignant disease. Interestingly, even with the high incidence of malignancy reported by Allo and Thompson,2 these investigators did not recommend preoperative needle biopsy; they believed that most occult tumors would be inaccessible to random biopsy. As noted, rarely, the thyroid carcinoma may be clinically evident with vocal cord paralysis or tracheal invasion. In these cases biopsy is indicated prior to any final therapeutic decision.
at the time of the excision of the substernal goiter. Torre and coworkers115 reported an incidence of 6.7% of cancer in their 237 cases of substernal goiter, but only 3 cases were of the occult type (1.2%). Of the other 13 patients with cancer, most had advanced, obvious clinical malignant disease. Interestingly, even with the high incidence of malignancy reported by Allo and Thompson,2 these investigators did not recommend preoperative needle biopsy; they believed that most occult tumors would be inaccessible to random biopsy. As noted, rarely, the thyroid carcinoma may be clinically evident with vocal cord paralysis or tracheal invasion. In these cases biopsy is indicated prior to any final therapeutic decision.
Symptoms and Signs
Most patients with substernal goiters are >50 years of age, with many in the seventh or eighth decade of life. Women are affected three to four times more often than men. The patients tend to be stout and thick-necked. Some degree of kyphosis is not uncommon. A variable number of patients report a history of having undergone one or more previous thyroid operations. In the series of Katlic44 and Sanders89 and their associates, the incidence was 20% exclusive of those patients with clinically suspicious malignant lesions. Patients with small extensions of a primarily cervical goiter present with a mass in the neck; the features are not dissimilar to the complaints and findings in patients with a purely cervical goiter. Patients with partial or complete intrathoracic goiters may be asymptomatic. According to Rietz and Werner78 as well as Rieve79 and Lamke48 and their colleagues, 17% to 32% of the patients with intrathoracic goiters are in this category, and the lesion is discovered only on routine chest radiography. Most patients, however, present with one or more complaints of a cervical mass, dysphagia, dyspnea, stridor, cough or wheezing, and facial flushing. Acute tracheal obstruction with severe respiratory compromise may occasionally be observed. The precipitating event may be obscure, but an acute respiratory infection may play a role. Le Roux and associates49 reported that the administration of iodine-131 (131I) may temporarily cause enlargement of the goiter and exaggerate any preexisting tracheal compromise. Acute airway obstruction as reported by Warren121 may lead to sudden death; he observed one death in four patients who developed this complication. The management of this emergent event has been discussed in detail by Shaha and associates93; essentially, an adequate airway must be established by intubation and urgent thyroidectomy performed.
Allo and Thompson2 reported that some large multinodular substernal goiters may result in incipient or frank thyrotoxicosis because of autonomously functioning hot nodules or because of the total bulk of functioning thyroid mass. In their series of 50 patients, thyrotoxicosis was present in 20%. Torre and associates115 noted an incidence of 13.1%. In many of these patients the thyrotoxicosis was manifested by cardiac failure, cardiac arrhythmia, or a wasting syndrome (apathetic thyrotoxicosis). In contrast to this high incidence of thyrotoxicosis, Shaha and associates92 reported in their series that only 1 of 60 patients with benign substernal goiters had thyrotoxicosis—an incidence of 1.6%.
Except for those few patients with a complete intrathoracic goiter or those with previous thyroid surgery (over 50% in the series of Ellis and colleagues28), the signs consist of a cervical mass, obesity in many, and obvious tracheal deviation in the neck. The cervical mass may move with swallowing. An infrequently encountered goiter, the so-called goitre plongeaut, is one that is normally nonpalpable in the neck but ascends into that area and becomes palpable when the patient coughs, swallows, or performs Valsalva’s maneuver. Shocket and Hudson99 suggested that hyperextension of the neck during Valsalva’s maneuver would facilitate the palpation of such a plunging goiter. Superior vena cava obstruction is seen in a small percentage (1%) of patients, even though almost all intrathoracic goiters are benign lesions. The mechanism of development most often is compression of the innominate veins and internal jugular veins against the bony margins of the thoracic inlet by the enlarged gland rather than compression of the superior vena cava per se. The dilated, enlarged veins in the neck have been referred to as Stokes’ collar, so named by that Irish physician in the nineteenth century. Compression of the superior vena cava, however, does occur when it is distorted by a large complete intrathoracic goiter in the anterior mediastinal compartment, as confirmed by Rodriguez and colleagues.80 The actual incidence is unknown.
In most series, evidence of malignancy has been absent, and the few tumors that have been discovered have been occult in nature. Exceptions do occur; this has been evident in the series reported by Allo and Thompson2 and Sanders and associates89 at the Lahey Clinic. Hoarseness caused by a paresis of a vocal cord is a strong indication of the possibility of a malignancy, but it may occur in the presence of a benign lesion as well, so that even though this finding is suggestive of the presence of a malignant tumor, it is not diagnostic.
Rarely, a large substernal goiter may cause a Horner’s syndrome, as recorded by Cengiz and associates.14 Even the occurrence of a cerebral steal syndrome was observed by Gadisseux and colleagues30 in a patient with a large substernal goiter. One of the rarest manifestations recorded was a partial hemidiaphragmatic paralysis due to pressure on one of the phrenic nerves by a recurrent substernal goiter. This case was reported by van Doorn and Kranendonk.118 Removal of the recurrent substernal goiter results in complete return of normal hemidiaphragmatic function.
Diagnostic Procedures
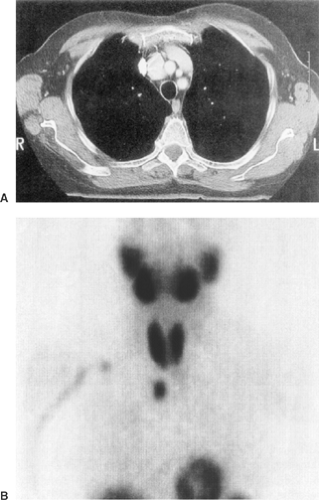
Standard radiography of the neck and chest is most often diagnostic. CT scans are frequently used when the goiter is a partial or complete intrathoracic goiter, particularly when it is posterior or retrotracheal in location. Radionuclide scintigraphy is also frequently used, and some investigators, such as Park and colleagues,70 believe it should be used routinely in all suspected cases. Needle biopsy is rarely indicated.
Radiographic Features
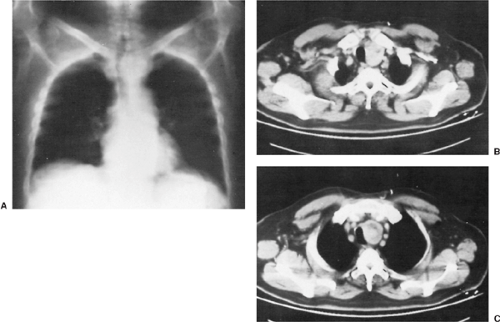
The standard radiographic finding in most cases is a mass to the right or left of the trachea in the anterosuperior portion of the visceral compartment. Tracheal deviation is present in 80% to >95% of patients. In contrast to other lesions that may cause tracheal deviation within the thorax, characteristically the deviation caused by the goiter begins in the cervical portion of the trachea (Fig. 175-3). Retrotracheal (posterior) goiters may deviate the trachea anteriorly and the esophagus posteriorly (Fig. 175-4).
Retroesophageal goiter displaces the esophagus anteriorly and laterally as well. This is best demonstrated on barium swallow. On lateral radiographs of the chest, the anterior cardiac window remains clear, and a density obscuring this area, which is characteristic of anterior mediastinal compartment tumors (e.g., thymic epithelial tumor [thymoma], lymphoma, or germ cell tumor), is absent. Calcification within the mass is variable. Katlic and associates44 reported only a 3% incidence of calcification; however, most studies, such as those of McCort59 and Ellis and colleagues,28 report much higher incidences of between 25% and 38%. On fluoroscopic examination, movement of the mass may frequently be observed on swallowing.
Computed Tomography
Computed tomography (CT) may reveal additional features to support the diagnosis. Bashist3 and Glazer34 and their coworkers reported (a) a clear connection or continuity between the intrathoracic mass and the cervical thyroid gland; (b) well-defined borders; (c) punctate, coarse, or ring-like calcifications in most masses; (d) nonhomogeneity with discrete nonenhancing low-density areas; and (e) prolonged contrast enhancement of the gland with iodinated urographic contrast material. Buckley and Stark11 noted that, on nonenhanced scans, the gland has a higher attenuation (100 Hounsfield units) than the adjacent tissues. With contrast enhancement, the attenuation is increased rapidly and is prolonged well beyond that of any adjacent tissues. Displacement of the trachea and esophagus is especially well demonstrated (Fig. 175-5) in the posterior goiters. This feature was well documented by the aforementioned researchers as well as by Morris and associates.64 In addition, with the use of an intravenous contrast bolus, the typical anterolateral displacement of the great vessels is readily demonstrated (Fig. 175-6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree