Chapter 101 Left Ventricular Restoration
Surgical Treatment of the Failing Heart
In response to the increasing health, economic, and social impacts of heart failure, clinicians are investigating new surgical therapies that do not involve heart transplantation. Heart failure affects about 5 million U.S. patients, and more than 250,000 die annually. Nearly 70% of patients with heart failure have coronary artery disease, and nearly all have had a myocardial infarction (MI).1 In part, the increase in the prevalence and incidence of heart failure results from improved diagnosis and treatment of cardiac disease (especially ischemic disease), but another factor is the aging of the population.2 Despite improvements in medical treatment since the 1960s and the introduction of potent new drugs, the prognosis for patients affected by heart failure remains extremely poor.3,4 However, with recent advances in surgical therapy for cardiac disease, new surgical options can be offered to some of these patients as an alternative to medical therapy. Some conventional drug therapies have not shown any significant improvement in the survival of patients with heart failure. For example, the Survival Trial of Amiodarone Therapy in Congestive Heart Failure (STATCHF) found no difference in survival among patients in heart failure who received amiodarone therapy or placebo.5
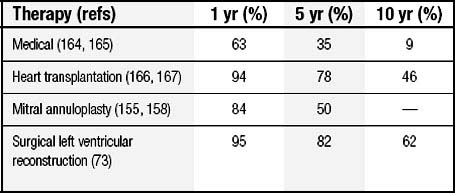
After diagnosis, despite improvements in medical management, 5-year mortality is 60% for men and 45% for women. For patients with late-stage disease (New York Heart Association [NYHA] class III and IV), even with maximal management, 1-year mortality is at least 20%; by 10 years, survivors are rare (Table 101-1).6–8
Historically, patients with severe congestive heart failure (CHF) were listed for heart transplantation, and although this is a very effective therapy for end-stage heart failure, the limited number of donor organs remains a crucial problem.9 Indeed, the Registry of the International Society for Heart and Lung Transplantation shows that the number of heart transplant centers worldwide has dropped from 248 in 1995 to 201 in 2005.10
Surgical management, until recently, was directed toward the underlying pathology of coronary disease and the secondary mitral insufficiency that evolves as the heart dilates, but surgeons do not systematically approach the ventricle. Surgical ventricular restoration (SVR), the topic of this chapter, was launched by Dor and colleagues11 and represents a relatively novel surgical approach that aims to restore (i.e., bring back to normal) the dilated, distorted left ventricular (LV) cavity to improve function. It requires knowledge of the remodeling infrastructure, of the structural changes that lead to geometric abnormalities, and of the role of compensatory, remote muscle and stretching mechanisms that lead to electrical instabilities.12
SVR is more than a single procedure, as it includes coronary grafting and mitral repair when needed, and thus it has the potential to treat the three components of the disease: the ventricle, the vessels, and the valve (“triple V” as defined by Buckberg13,14).
PATHOPHYSIOLOGY OF HEART FAILURE
Left Ventricular Remodeling
In dilated cardiomyopathy (a common cause of heart failure), a primary determinant of the disease process is LV remodeling. The underlying causes of dilated cardiomyopathy are diverse, and the origin may be nonischemic or ischemic.
LV remodeling is a complex, dynamic, and time-dependent phenomenon that involves molecular, cellular, interstitial, and genome expression changes that manifest clinically as changes in the size, shape, and function of the heart after cardiac injury.15 The process can evolve slowly or rapidly after the myocardial injury, and it contributes importantly in the progression to end-stage CHF. Box 101-1 summarizes the principal changes that occur in LV remodeling. The extracellular matrix participates in the altered ventricular geometry after MI. Cardiologists and cardiac surgeons traditionally viewed the extracellular matrix as an inert collection of structural macromolecules that serve as a scaffold for cells. However, a large body of evidence supports a central role for the extracellular matrix in the control of numerous cellular functions16 and supports the idea that the extent of LV remodeling is a critical determinant of clinical outcome after MI.
Current working models for heart failure include the cardiorenal model (excessive salt and water retention), the hemodynamic model (pump failure and excessive vasoconstriction), and the neurohormonal model (overexpression of biologically active molecules capable of exerting unfavorable effects on the heart and circulation). Each of these may be necessary but not entirely sufficient to explain all the causes of disease progression in the failing heart. After the myocardial insult and the initial decline in pumping capacity, a variety of compensatory mechanisms are evoked to restore cardiovascular function and normal homeostasis. Adrenergic, renin-angiotensin, and cytokine systems are activated systemically and locally in the myocardium in response to reduced cardiac output.17,18 A variety of circulating and tissue proteins and peptides (e.g., norepinephrine, angiotensin II, endothelin, aldosterone, tumor necrosis factor, interleukins) are generated in an initial adapting response. However, chronic overexpression of these biologically active molecules may fundamentally alter gene expression, changing protein synthesis in both myocytes and fibroblasts.
The biomechanical model for heart failure described by Mann and Bristow helps to explain the progression of heart failure independent of the neurohormonal status of the patient.19 In fact, current medical therapy, acting against neurohormonal activation, tends to slow progression but fails to arrest the process of remodeling. In addition, many types of neurohormonal inhibition proved to be ineffective or even harmful in patients with heart failure.20
To explain the failure of neurohormonal antagonism, Mann and Bristow focus on LV size and geometric abnormalities as being responsible for progression of the disease. Geometric changes lead to structural abnormalities of the myocytes and of the myocardium, which worsen cardiac function and increase neurohormonal activation; this may make the cardiovascular system less responsive to normal homeostatic control mechanisms.19
Left Ventricular Geometric Abnormalities in Ischemic and Nonischemic Ventricles
The determinants of LV geometry are shape, volume, and cardiac mass, and these three components may be altered by cardiac disease, which can be primarily degenerative, valvular, or ischemic in origin. Ischemic cardiomyopathy leads to a sequence of structural changes to compensate for the increased load produced from the nonfunctional akinetic or dyskinetic regions.21
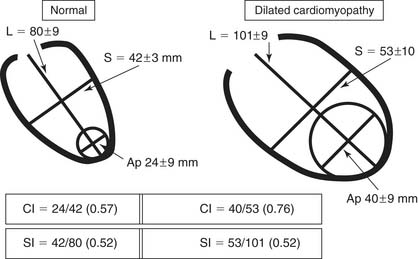
In anterior myocardial infarction, the LV apex is primarily involved, so regional changes affect the anterior, septal, and inferoseptal ventricular components. Globally, the elongation and widening of the ventricle occur proportionally so as to maintain a constant ratio (i.e., a constant sphericity index, or short ratio to long axis). However, when patients develop secondary mitral insufficiency, the sphericity index is abnormal and the ventricle is more spherical. In the absence of mitral regurgitation (MR), therefore, the sphericity index fails to detect shape abnormalities in anterior postinfarction cardiomyopathy. The conicity index has been proposed to assess the conical shape of the apex and its changes after MI (Fig. 101-1).22 Another important shape change that occurs after an anterior MI is the displacement of the papillary muscles laterally and toward the apex, which produces tethering of the posterior mitral leaflet and mitral restriction.
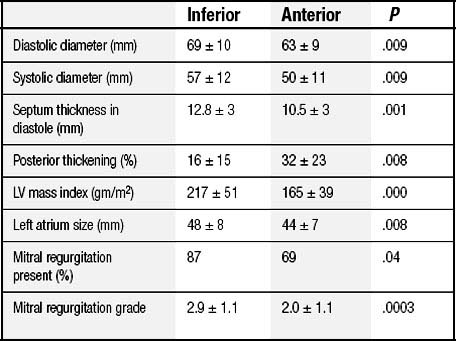
On the other hand, shape changes after an inferior MI are different23: the short axis is widened more than the long axis is elongated, which leads to an increase in the sphericity index and accounts for the more frequent occurrence and the more severe degree of MR. Table 101-2 compares the geometric abnormalities that occur in anterior and inferior myocardial infarctions.24
Nonischemic dilated cardiomyopathy exhibits a more spherical shape than ischemic cardiomyopathy: the short axis is enlarged, MR is more frequently involved, and wall motion is severely and diffusely hypokinetic. Pump function is markedly reduced and there are apparently no regional differences in contractility. However, biopsy studies show nonhomogeneous disease in many patients, with the amount of scarring and fibrosis ranging from 4% to 60% between the free wall and the septum, which may account for the failure of some surgery, such as the Batista operation, to reduce the ventricular cavity in patients with nonischemic dilated cardiomyopathy.25,26
The Rationale for Reshaping Cardiac Architecture
Figure 101-2 shows the spiral arrangement of myocardial fibers as reported in a very old anatomy atlas. Studies27,28 established that fiber orientation was a function of transmural location, with fiber direction being predominantly longitudinal in the endocardial region, transitioning into a circumferential direction in the mid wall, and becoming longitudinal again over the epicardial surface. Such an alignment of fibers in double layers (left- and right-handed from the base to the apex) forms a double helix. These layers are not aligned parallel to one another; rather, the myofiber sheets diverge within the LV wall, creating angulations with respect to the plane of the epicardial surface. These fiber angulations serve to resist deformation and to maintain the distribution of tension within normal limits at the three levels: longitudinal, radial, and circumferential. Moreover, this architecture gives to the heart a form resembling a geometric ellipsoid, which favors the direction of flow toward the aorta. When angulations are deformed by fiber disruption, fibrosis, or scarring of myocardial tissue, the shape of the ventricle loses its characteristics and its functionality (following the strict relationship between form and function). For a given fiber contractile status, the ejection fraction (EF) changes according to the shape of the ventricle, being low in a spherical ventricle (sphericity index, toward 1) and high in an elliptical shape (sphericity index, toward 0). In fact, macroscopic anatomic alterations can affect wall tension, which, according to the Laplace law, is directly proportional to LV pressure and chamber radius and inversely proportional to wall thickness. In animal models of myocardial damage or after MI in humans, ventricular remodeling alters the major determinants of wall stress,29,30 and functional impairment has been reported in the remote zones of dilated LV with geometric abnormalities.31 When the ischemic systolic dysfunction is superimposed on the use of preload to improve cardiac output during exercise, the ventricle can be further dilated because of its inability to eject blood, starting a cycle that further deteriorates LV function. In addition, LV end-diastolic pressure can increase in the presence of reduced myocardial compliance (resulting from tissue stiffening), which tends to resist deformation, whereas increased stiffness leads to decreased systolic function.
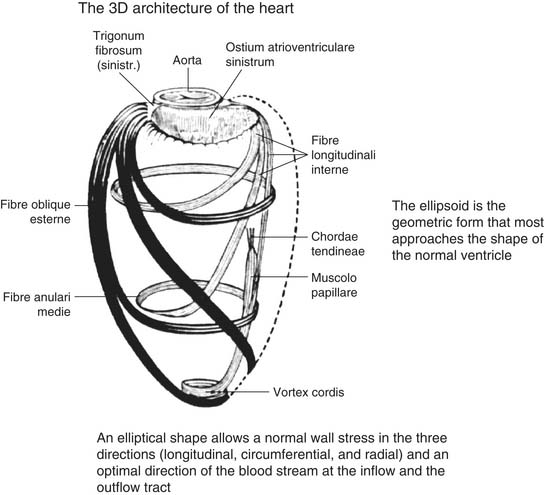
Figure 101–2 From an 18th-century atlas of anatomy
(From Benninghoff-Goertler, Atlas of Anatomy, vol II, 1996, Piccin Editor.)
A further increase in end-diastolic and end-systolic volumes leads to an adverse prognosis.32 As part of the GUSTO (Global Utilization of Streptokinase and t-PA for Occluded Arteries) trial, Migrino and associates32 evaluated end-systolic volume at 90 to 180 minutes into reperfusion during acute MI. They demonstrated that in successfully reperfused patients after MI, an increase in end-systolic volume beyond 40 mL/m2 worsens prognosis in terms of both development of heart failure and mortality. A post-MI end-systolic volume index equal to or greater than 60 mL/m2 carries a 1-year mortality rate of up to 30%. In the SAVE (Survival and Ventricular Enlargement) trial, left ventricular size was a strong independent risk factor for mortality after 2 years.33
Even after coronary artery bypass surgery, patients with large ventricles have a worse prognosis. Yamaguchi and coworkers34 identified LV end-systolic volume index greater than 100 mL/m2 as an independent risk factor for the development of CHF in ischemic cardiomyopathy. These reports helped cardiac surgeons focus on the importance of reconstruction of the left ventricle in ischemic cardiomyopathy.
Left Ventricular Aneurysm or Ischemic Dilated Cardiomyopathy?
Left ventricular aneurysm was first described in the 18th century.35 In 1816, Cruveilhier36 attributed ventricular aneurysm to myocardial fibrosis, although its association with coronary thrombosis was not generally appreciated until a century later. It was not until Tennant and Wiggers showed paradoxical motion in acutely ischemic myocardium that the physiologic implications of ventricular injury became apparent.37 Subsequently, Murray described systolic paradoxical expansion of acutely infarcted myocardium and correlated this with diminished cardiac output and falling blood pressure.38 In 1967, Klein, Gorlin, and coworkers39 published a hemodynamic study on LV aneurysm that became a milestone in the field of mechanics and energetics of the left ventricle after an ischemic injury. Gorlin and coauthors were the first to state that when approximately 20% to 25% of left ventricular area is inactivated by any pathologic process, the degree that the myofiber must shorten to maintain stroke volume exceeds physiologic limits, and cardiac enlargement (Starling mechanism) must ensue to maintain adequate ejection of blood. With this concept, they anticipated the concept of LV remodeling and described the way MI relates to the genesis of aneurysm. Furthermore, they described that the aneurysm can be either dyskinetic (i.e., paradoxical expansion resulting during systole) or akinetic (i.e., myocardial fibrosis, calcification within the scar, thickened overlying pericardium, mural thrombosis, and endocardial thickening may rigidify the aneurysm wall and prevent its expansion).
Gorlin offered the following definition:
An aneurysm is identified by a left ventricular angiogram as any akinetic or dyskinetic segment of myocardium. An akinetic segment is defined as a segment that appears to have no motion during systole, whereas a dyskinetic segment appears to bulge paradoxically during systole. Intraoperatively, an aneurysm is identified as a circumscribed area of scar, which is thin, often adherent to the pericardium and which may or may not bulge paradoxically during systole. The aneurysmal segment is easily outlined by looking for the area that puckers and collapses when the left ventricle is vented.
This cineangiographic and surgical definition of ventricular aneurysm is compatible with the pathologic definition: “A localized outpouching of the cavity of a cardiac chamber, with or without outward bulging of the external surface.”40
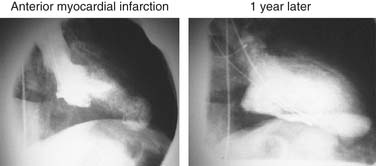
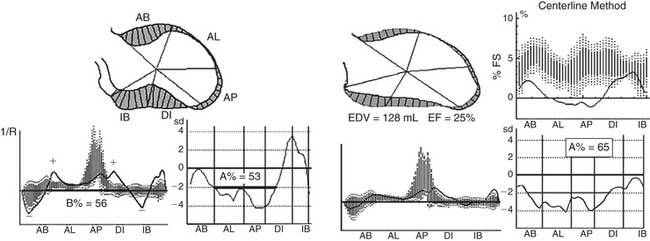
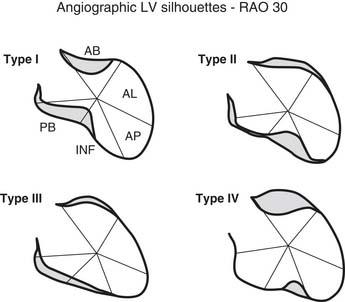
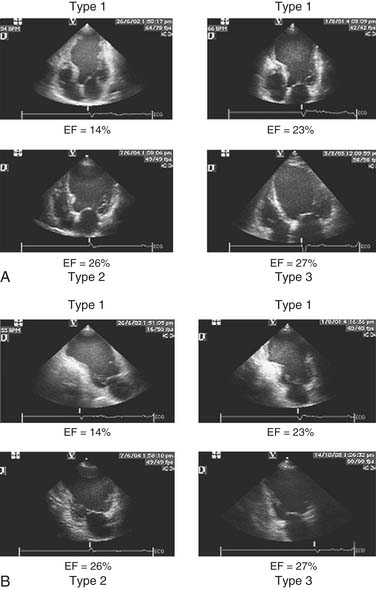
Early revascularization, with either thrombolysis or primary percutaneous transluminal coronary angioplasty, has beneficial effects on infarct size and LV function and therefore has profoundly changed the picture of MI and its sequelae.41–43 However, even with early mechanical relief of the coronary occlusion, unfavorable global LV remodeling may occur. Bolognese and coauthors demonstrated that almost 30% of patients with excellent infarct-related artery patency at 6 months continue to undergo LV remodeling.44,45 In patients with an anterior MI, this occurs more frequently when myocardial contrast echocardiography demonstrates a higher incidence of microvascular dysfunction. Figure 101-3 shows the progression of an anterior MI toward ischemic dilated cardiomyopathy in one of our patients. Figure 101-4 shows two of the shape abnormalities that may occur after successful early recanalization for anterior MI as analyzed by the centerline method.46–48 Thus, an acute MI, either anterior or inferior, may result in any of four types of shape abnormalities (Fig. 101-5).49 This classification is incomplete because it does not take into account abnormalities occurring at the septum or at the lateral wall. However, it is useful for assessing treatment results, especially after volume reduction surgery, when the results after repair of a true aneurysm look profoundly different from the results after surgery for type 2, 3, or 4. Figure 101-6 shows an echocardiographic study from four patients with three types of shape abnormalities. Apical four-chamber and two-chamber views are shown.
Left Ventricular Aneurysmectomy
The first successful surgical correction of an LV aneurysm occurred in 1957.50 Denton Cooley described the technique of open resection and simple closure on cardiopulmonary bypass in 1958.51 This technique was the standard of the profession for the next 30 years. In 1968, Favaloro and colleagues52 reported on a series of 130 patients who underwent resection for LV aneurysm with a hospital mortality of 13%.
In 1979, Grondin and associates53 reported on a series of 40 patients with aneurysms who were medically managed. They divided the group into those with and those without symptoms. After 10 years, survival was 90% in asymptomatic patients but only 46% in patients who were symptomatic at the time of diagnosis. The causes of death were predominantly CHF, thromboembolism, and arrhythmias. They suggested that mortality depended on aneurysm size, with large aneurysms conferring a higher risk. These reports implied that medical management resulted in improved survival, and they fostered a reluctance to use surgery to treat this disease. As recently as 1983, Cohen and coworkers54 recommended that aneurysms be resected only after maximal medical management (for heart failure, angina pectoris, recurrent thromboembolism refractory to anticoagulation, and refractory ventricular tachycardia) had failed.
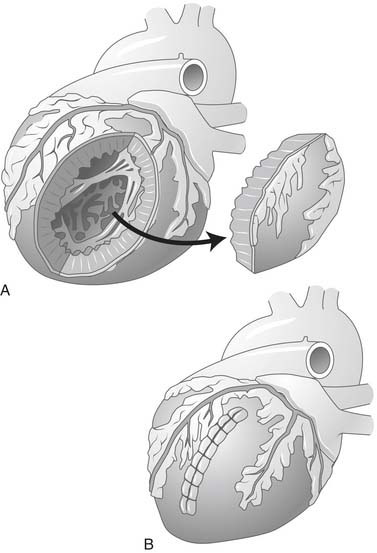
In 1985, the concept of surgical repair of the LV aneurysm was altered. Newer techniques described by Jatene55 involved excluding the dyskinetic scar when performing a circular endoventricular suture, and Dor and colleagues11 began using an endoventricular patch to rebuild a failing ventricle after extended endocardectomy for ventricular tachycardia. The concept was to reduce the LV size and reconstruct a more elliptical cavity, treating the dilation in all its components (anterior, apical, and septal), as opposed to performing a linear resection56,57 of the aneurysm, which left untouched a septal dilation and created a distortion of the residual chamber. The concept of excluding all diseased tissue from the cavity was a fundamental improvement.11 With this change in technique, a reduction was seen in hospital mortality as well as in late mortality. Dor and coworkers demonstrated that the technique is applicable not only to dyskinetic but also to akinetic areas. As a consequence, the indications and patient selection for volume reduction surgery have changed.58
DIAGNOSIS AND PATIENT SELECTION FOR SURGERY
Nonischemic Cardiomyopathy
LV reconstruction for dilated cardiomyopathy (the Batista procedure, or partial left ventriculectomy) has been abandoned, primarily because of unacceptable perioperative mortality and morbidity.26 One reason for its failure was that the pathology of the whole chamber was assumed to be uniform and homogeneous. However, there is evidence that the disease is nonhomogeneous, and that the septal and lateral walls differ in scarring and fibrosis.59,60 Thus, in the Batista operation, if a minimally diseased lateral wall was excised and a very fibrotic septum was retained, postoperative LV function was adversely affected, even if the left ventricle was properly downsized.
Therefore, Suma and colleagues60 suggested an intraoperative echocardiographic evaluation to assess the regional contractile response to preload reduction, to aid site selection, and to improve surgical results. In the intraoperative echocardiography-guided volume reduction test, the initiation of partial cardiopulmonary bypass decompresses the dilated (stretched) chamber and induces functional changes of left ventricular wall motion and thickness. Identification of wall motion changes means that the most diseased region can be selected for exclusion, leaving the more viable muscle to resume function after restoration. This idea comes from experience in restoring the ischemic left ventricle, when the scar is evident and can be excluded. However, the concept of nonhomogeneity in nonischemic cardiomyopathy is new. Suma reports that if intraoperative echocardiography shows that the septum is the weakest part, the septal anterior ventricle is excluded. Septal anterior ventricular exclusion (SAVE) was introduced by Suma25,59,60 and called pacopexy by Buckberg and coauthors61 in recognition of the contributions of Francisco (Paco) Torrent-Guasp, whose ingenious anatomic concepts defined the helical ventricular myocardial band and furthered our understanding of the relationship between structure and function.13,62 To make an elliptical ventricle, a long and narrow endoventricular patch is placed along the septum with interrupted mattress sutures so that the septum and a part of the anterior wall are excluded (Fig. 101-7). In patients with nonischemic dilated cardiomyopathy and advanced NYHA class, results in terms of survival rate are promising.
When intraoperative echocardiography shows that the weakest part is the lateral wall, Suma and coworkers60 perform a partial left ventriculoplasty (Fig. 101-8).
Asynergic Areas
The left ventricle should be carefully evaluated by coronary angiography (ventricular angiography in right and left anterior oblique projections), by a complete echocardiographic study (four- and two-chamber views, and parasternal long- and short-axis views), or by a magnetic resonance imaging (MRI) study. The objectives of these imaging techniques are to assess parameters for patient selection (Box 101-2), treatment planning, and follow-up.
Patients with either akinetic or dyskinetic scar may benefit from SVR, and it is the extent of asynergy rather than the type of asynergy that is related to outcome after the surgery.63 The region to be surgically excluded should be carefully evaluated for wall motion and thickening. Wall motion assessment can be accomplished by LV angiography, echocardiography, or nuclear scintigraphy, but MRI provides a more accurate determination of LV volumes and EF, as it shows the epicardial and endocardial border from the base to the apex in a three-dimensional view.
LV angiography is a planar technique that shows at most two projections along the ventricle. It does not show the epicardial border, so it does not help in a calculation of thickening. However, application of the centerline method to LV angiography resulted in a reliable quantitative assessment of regional wall motion48 with clear definition of asynergy. Echocardiography allows visualization of both the endocardial and the epicardial borders, so wall thickening and wall motion can be assessed. However, an echocardiographic method that precisely defines regional wall motion is not available. Therefore, wall motion is measured by the wall motion score index,64 which results from a qualitative (thus subjective) evaluation of motion and is expressed by a number derived from the sum of the degree of asynergy at 16 different segments (normal, 1; hypokinetic, 2; akinetic, 3; dyskinetic, 4; aneurismal, 5).
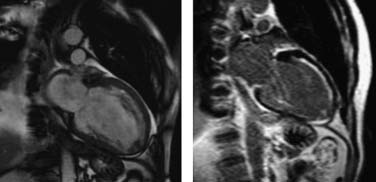
Nuclear scintigraphic methods display endocardial border motion in planar views (with radionuclide angiography, labeling the blood pool) or the myocardium, including endocardial and epicardial visualization with gated single-photon emission computed tomography. However, such studies require the use of radioactive tracers and are not available in all cardiac centers. Also, cardiovascular MRI is not available everywhere, and some patients have contraindications to the study (e.g., claustrophobia, presence of implantable cardioverter defibrillator). However, it allows a most comprehensive evaluation with highly accurate and reproducible measurements in a single session.65 The greatest usefulness of cardiovascular MRI is in the detection of myocardial scar with late gadolinium enhancement. Myocardial scar tends to accumulate a significantly higher concentration of gadolinium than normal myocardium. Ten to 20 minutes after infusion, scarred regions appear very bright, whereas normal myocardium appears dark, at typical imaging times. Figure 101-9 shows a patient with extensive scarring in the anterior and apical territory. A predictor of myocardial viability is the ratio of the thickness of tissue exhibiting late contrast enhancement in a segment to the total LV wall thickness in that segment. Segments with nearly transmural extent of late contrast enhancement are highly unlikely to have recovery of function after revascularization.66
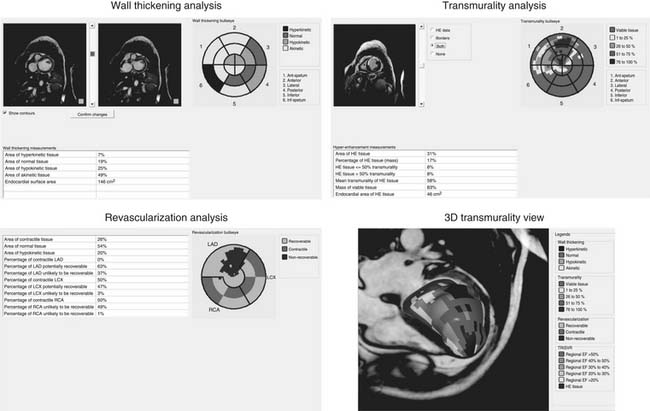
Commercially available software dedicated to LV function analysis now provides a semi-automated assessment of regional wall motion, the extent of normal and abnormal contracting myocardium, and the percentage of scarred tissue (Fig. 101-10). This allows a prediction of myocardial viability without a need for pharmacologic stress. Cardiovascular MRI also allows quantification of regional deformation by radionuclide tagging and calculation of strain and strain rate, which can be particularly useful for examining the remote regions and the apical twisting and untwisting.67 Torsion and twisting and untwisting movements of the apex (as opposed to the base) greatly contribute to blood ejection and to maintaining a good pump function; these mechanisms are reduced or even lost after an anterior MI, resulting in further impairment in cardiac function. Cardiovascular MRI tagging, however, is not routinely performed in clinical practice but is confined to research studies because the data analysis is laborious and time consuming and requires intensive computation. With the development of a tissue Doppler imaging technique and the two-dimensional (2D) “speckles” or endocardial border tracking analysis,68,69 it is easier to measure systolic and diastolic deformation either longitudinally or radially. Vector direction gives the clockwise and counterclockwise directions of contraction, which is particularly useful for assessing torsional mechanics at the apical level. An analysis of untwisting also provides information on diastolic function, at least on rapid filling.
Status of Remote Regions
The quantification of remote regions is performed to determine whether a patient is a candidate for an SVR procedure, and these regions are often not evaluated by conventional imaging studies. After an anterior MI, remote regions may show hypokinesia or even akinesia resulting from critical coronary disease in the right or left circumflex coronary artery (hibernating myocardium), or remote myocardium may be dysfunctional in the absence of coronary stenosis because of the high local tension that reduces shortening. Several years ago, we demonstrated31 that SVR induced an improvement in remote nonischemic regions in patients with anterior infarction; in that study, we excluded patients with significant stenosis in the right and left coronary arteries. Nowadays, with cardiovascular MRI, we can predict the recovery of function in ischemic, hibernating areas that will benefit from concomitant coronary artery bypass grafting (CABG), and we can also predict whether dysfunctional, nonischemic segments may recover after volume reduction. Therefore, we propose a term other than hibernating or stunned for dysfunctional nonischemic myocardium in dilated cardiomyopathy—exhausted myocardium—which implies recovery of function if the hemodynamic burden that imposes a high wall tension is relieved by SVR.
Finally, cardiologists, radiologists, and surgeons must collaborate to improve knowledge about patient selection and to achieve an optimal surgical outcome.
SURGICAL VENTRICULAR RESTORATION FOR ISCHEMIC CARDIOMYOPATHY
Left ventricular reconstruction by endoventricular circular patch plasty (EVCPP) repair was described and proposed by Dor and colleagues in 198411 for rebuilding the left ventricle after an MI, either during the acute phase (surgical treatment of septal rupture or refractory ventricular arrhythmias according to the Harken technique70) or in patients with chronic MI and with LV asynergy (akinesia or dyskinesia) to exclude all the akinetic nonresectable areas (e.g., septum and posterior wall). In addition, a complete coronary revascularization is achieved and mitral repair or replacement is performed if needed.71
Since the first description by Dor and colleagues,11 the procedure has been adopted by many surgeons, but it is not widely used because surgeons have been unwilling to incise and exclude the akinetic normal-appearing segments often encountered after early reperfusion. Instead, CABG is performed, and the nonfunctioning akinetic muscle containing deeper scar is left undisturbed.72
The technique has not been standardized, and surgeons use essentially four variations of LV reconstruction to exclude the septum. These include a linear closure by Jatene,55 a modified linear closure by Mickleborough,73 a circular closure with a patch by Dor and Menicanti,74 and a double cerclage closure without a patch by McCarthy.75 All of these techniques involve an incision into the diseased anterior wall, an exclusion of the entire diseased segment, and a reduction in ventricular cavity size. In the majority of patients, reconstruction is done on the anterior portion of the left ventricle. However, reconstruction has also been performed on the posterior wall after circumflex or right coronary artery occlusion. Most of these patients undergo concomitant coronary artery bypass, and many also undergo mitral valve repair.
Surgical Details of Anterior SVR
Surgical left ventricular restoration as performed since 2001 by our group at the San Donato Hospital in Italy is conducted on the arrested heart with antegrade crystalloid or cold blood cardioplegia. CABG is first performed, as completely as possible, almost always on the left anterior descending coronary artery, to preserve the upper part of the septum and to guarantee complete revascularization. The ventricle is opened at the middle of the scar on the anterior wall, with an incision parallel to the left anterior descending artery, starting from the midportion and proceeding toward the apex. The internal part of the cavity is examined, and thrombi are removed if present. The mitral valve is repaired, when necessary, through the ventricular opening with a double-armed stitch at the posterior annulus, from trigone to trigone, and the mitral orifice is undersized with a 26-mm valve sizer.76
In July 2001, we introduced the Mannequin (Chase Medical, Richardson, TX), which we fill with 50 to 60 mL of saline per square meter of the patient’s body surface area to optimize the size and shape of the new ventricle. This TRISVR technique is a refinement of the Dor technique that introduced a sizer device in 1998, which allows standardization of the procedure. The device is inserted into the LV cavity after an incision is created (Fig. 101-11), and then carefully inflated with saline when it is seated properly in the cavity, to avoid the risk of inflating the device when it is in the mitral valve. The new apex will be placed at the apex of the Mannequin, and this is the starting point for the 2/0 endoventricular circular suture. The suture is carried deep toward the septum and up toward the aortic outflow tract on an oblique plane to reconstruct an elliptical shape, not a spherical chamber. The suture is then brought toward the lateral wall, and back to the apex, and tightened on the Mannequin. The plane of the closure should not be parallel to the mitral valve (Fig. 101-12). If the closure plane is parallel to the mitral valve, the result is a spherical chamber (Fig. 101-13). The shape of the device is appositely conical with a physiologic short-to-long-axis ratio, so as to reconstruct a more physiologic, elliptical shape. After positioning the new apex, the surgeon places the circular stitches at the transitional zone (between the scarred and the sound tissue). When the ventricle is not very enlarged, the Mannequin reduces the risk of creating a residual cavity that is too small. It is also useful when the infarcted region is not clearly demarcated, as occurs in dilated cardiomyopathy (type III silhouette [see Fig. 101-5], as described by our group).49 In this circumstance, the transitional zone between scarred and nonscarred myocardium is not well defined, and the Mannequin allows rebuilding of the ventricle in an elliptical way with a proper residual size. Box 101-3 shows surgical pitfalls during SVR.
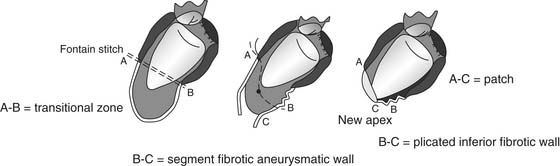
Figure 101–11 The use of the intraventricular Mannequin during surgical left ventricular reconstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree