Left Ventricular Dyssynchrony and Cardiac Resynchronization Therapy
David Spragg
Ronald Berger
David Kass
Hugh Calkins
Congestive heart failure (CHF) is an increasingly common disorder. The incidence of CHF in the United States is more than 550,000 cases per year, with an estimated annual mortality of over 300,000 (1). The pathogenesis of CHF is complex, arising from the combined effects of compromised myocardial function, neurohormonal signaling cascades, and, in many cases, disordered mechanical ventricular activation. Left bundle branch block (LBBB), a surrogate marker for left ventricular mechanical dyssynchrony, is present in between 25% to 50% of patients with CHF and is associated with a substantial increase in morbidity, mortality, and sudden cardiac death in CHF patients (2,3,4).
More than 10 years ago, the first case report of multisite pacing to recoordinate a failing, dyssynchronous left ventricle (LV) was published (5). In the interim, cardiac resynchronization therapy (CRT) has become an increasingly widespread and important strategy in the treatment of patients with severe CHF. Recent large-scale, randomized clinical trials have demonstrated that CRT can reduce both morbidity and total mortality in select populations of CHF patients. This chapter reviews the pathobiology of LV dyssynchrony, as well as the acute and chronic effects of cardiac resynchronization. The major, recent trials investigating the efficacy of CRT in patients with heart failure (HF) are reviewed, as are the guidelines governing CRT implementation derived from those trials. The technical aspects of CRT device implantation are discussed briefly. Finally, unresolved issues and controversies surrounding patient selection and screening for CRT are considered.
Pathology of Left Ventricular Dyssynchrony
LV dyssynchrony typically results from delay in the activation of the lateral LV free wall and is manifest frequently (but not necessarily) by LBBB on surface electrocardiogram (ECG). Contraction of the septum and anterior LV in early systole results in prestretch of the still-quiescent lateral wall, delaying intracavitary pressure rise and mitral valve closure. Late-systolic activation of the LV lateral free wall leads to corresponding stretch of the anteroseptal region, thereby competing with aortic ejection and reducing net cardiac output. The result is mechanical inefficiency, with transmission of the ventricular blood pool between two intracavitary sinks (the stretched lateral wall in early systole, and the anteroseptal region in late systole). Functional mitral regurgitation, which is due to delay in both the rise in LV intracavitary pressure and discoordinate papillary muscle contraction, can exacerbate this inefficiency further.
LV dyssynchrony results in an array of pathological changes (Table 75.1) Globally, systolic ventricular function is immediately compromised with the onset of disordered ventricular activation (6,7,8). Locally, regions of delayed myocardial activation are subject to increased fiber strain and work, with parallel increases in myocardial blood flow, metabolic activity, and tissue hypertrophy (Fig. 75.1A,B) (9,10,11). Electrophysiological properties of high-strain, late-activated myocardium in dyssynchronous hearts are deranged, with zones of reduced conduction velocity and reduced action potential duration and tissue refractoriness (12). Finally, late-activated myocardium in dyssynchronous, failing hearts has been shown to undergo a variety of changes in protein expression (Fig. 75.1C), including reduction in the levels of calcium-cycling proteins, including sarcoplasmic reticular calcium ATPase2a (SERCA2a) and phospholamban (PLB), reduction in expression of the gap-junction protein connexin43 (Cx43), and increased local activation of the stress response kinase extracellular-signal regulated kinase (ERK42/44) (13).
Effects of Cardiac Resynchronization Therapy
The mechanical and energetic consequences of intraventricular dyssynchrony can be mitigated by either biventricular or LV-only pacing. In both cases, early stimulation of the lateral LV free wall results in recoordination of ventricular contraction
(Fig. 75.2A) (14). The effects of ventricular resynchronization on LV mechanical work are instantaneous, with appreciable increases in dP/dtmax, aortic systolic pressure, and cardiac output (CO) occurring within one beat of LV pacing onset (Fig. 75.2B). Stroke volume (width of the pressure-volume loop) increases acutely, with an attendant decline in end-systolic stress (Fig. 75.2C). Underlying these laudatory effects on global LV systolic function is the elimination of early- and late-systolic stretch in the lateral and anteroseptal LV walls, respectively. Rather, both territories contract throughout systole (Fig. 75.2D).
(Fig. 75.2A) (14). The effects of ventricular resynchronization on LV mechanical work are instantaneous, with appreciable increases in dP/dtmax, aortic systolic pressure, and cardiac output (CO) occurring within one beat of LV pacing onset (Fig. 75.2B). Stroke volume (width of the pressure-volume loop) increases acutely, with an attendant decline in end-systolic stress (Fig. 75.2C). Underlying these laudatory effects on global LV systolic function is the elimination of early- and late-systolic stretch in the lateral and anteroseptal LV walls, respectively. Rather, both territories contract throughout systole (Fig. 75.2D).
TABLE 75.1 Pathophysiological Consequences of LV Dyssynchrony | ||
|---|---|---|
|
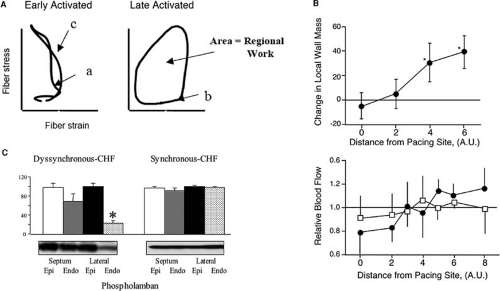 FIGURE 75.1. A: Stress-strain loops from early- and late-activated myocardial regions in dyssynchronous hearts (9). In early-activated regions, contraction initially occurs at low stress levels (a) as quiescent, late-activated regions undergo passive stretch. Later in systole, early-activated regions undergo reciprocal deformation as the late-activated territories contract (c). The small net area of the stress-strain loop in early-activated regions reflects reduced regional work performed. In late-activated territories, passive stretch in early systole generates increased stress before contraction (b). The increased stress-strain loop area reflects increased work performed by late-activated territories. B: Sustained ventricular pacing leads to hypertrophy at regions distant from the site of pacing and to increased myocardial blood flow (11) in regions distant from the pacing site (solid circles). With chronic pacing, regional changes in myocardial blood flow return equilibrate (open squares). C: Certain changes in protein expression, including downregulation of phospholamban, appear to be uniquely confined to late-activated lateral LV endocardium of dyssynchronous, failing hearts (13); similar changes in protein expression are not seen in any region of hearts with equivalent failure but preserved systolic function. |
The beneficial effects of mechanical LV resynchronization appear to be independent of whether electrical synchrony is concomitantly achieved. Comparisons of LV-only to biventricular pacing have shown that although LV-only pacing did not improve (and actually worsened) electrical dyssynchrony across the LV, the mechanical effects of LV and BiV pacing schemes were essentially identical (14). Both modes increased dP/dtmax, CO, and stroke volume. More recent work has revealed some differences between LV and biventricular pacing, although these differences appear to be confined to diastole. In clinical studies of the two pacing strategies in patients with dyssynchronous heart failure, BiV pacing improved isovolumic relaxation rates, whereas LV-only pacing did not (15,16,17). This disparity may reflect differences in the duration of myocardial activation between the two pacing patterns, with BiV activation leading to more rapid contraction and earlier relaxation. When the LV alone is paced, contraction is longer in duration and thus compromises the diastolic period and relaxation rates.
Chronic ventricular resynchronization with biventricular or LV-only pacing results in further improvement of LV function and induces reverse remodeling in patients with dilated cardiomyopathy (18,19,20,21,22). In a study of 25 patients with class III-IV CHF and QRS widths of greater than 140 ms, for instance, Yu et al. demonstrated reductions in both end-systolic and end-diastolic volumes after 3 months of chronic biventricular pacing (18). With cessation of pacing, the investigators
found that dP/dtmax immediately declined (acute CRT effect), but chamber volumes were not acutely altered (Fig. 75.3A,B). This supports a remodeling effect rather than an active effect of CRT itself on chronic chamber volumes. Subsequent studies including the MIRACLE (23) and Vigor-CHF (24) trials have reported approximately 10% reductions of both end-systolic and end-diastolic volume with 6-month CRT treatment.
found that dP/dtmax immediately declined (acute CRT effect), but chamber volumes were not acutely altered (Fig. 75.3A,B). This supports a remodeling effect rather than an active effect of CRT itself on chronic chamber volumes. Subsequent studies including the MIRACLE (23) and Vigor-CHF (24) trials have reported approximately 10% reductions of both end-systolic and end-diastolic volume with 6-month CRT treatment.
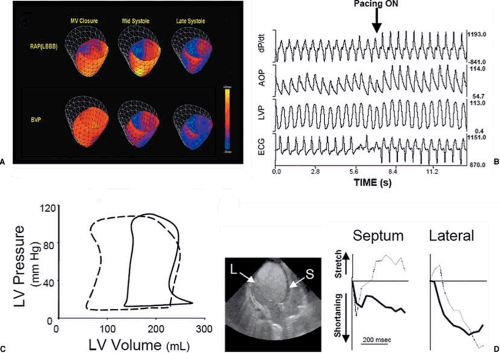 FIGURE 75.2. A: Tagged magnetic resonance imaging maps showing regional wall deformation during systole in an LBBB model during RA pacing (top panel) and BiV pacing (bottom panel). Transition from red to blue indicates shortening; transition from red to yellow indicates stretch (14). B: The acute hemodynamic effects of CRT on dP/dtmax, aortic pressure, and LV pressure in a dyssynchronous human LV. Onset of CRT is accompanied by instantaneous rises in all parameters, including an increase in aortic pulse pressure (consistent with enhanced cardiac output). C: PV loops displaying the effects of CRT. Resynchronization induces a left shift of the entire loop, with increased stroke volume and reduced end-diastolic filling pressures. D: Regional wall motion before (light lines) and during (heavy lines) CRT therapy. With dyssynchrony, the septum undergoes early systolic contraction and late systolic stretch (as the lateral wall contracts); the lateral LV is stretched in early systole, and then shortens. Initiation of CRT induces consistent shortening in both regions throughout systole. |
In contrast to therapy with positive inotropes, the increase in systolic function obtained from CRT in dyssynchronous failing hearts does not increase (and may actually reduce) myocardial oxygen consumption. Nelson et al. first reported on the energetics of a traditional inotropic treatment (dobutamine) versus CRT in dyssynchronous LV failure (25). They found that equivalent increases in dP/dtmax were associated with marked differences in oxygen use, with O2 consumption (per beat) rising nearly 20% with dobutamine, but falling by 10% with CRT (Fig. 75.3C).
The impact of CRT on the electrophysiological or molecular changes reported to occur with chronic dyssynchrony is unknown. Some have expressed concern that the use of an epicardial pacemaker (i.e., the LV lead in a CRT system) is arrhythmogenic because of differences in transmural conduction and repolarization (26). However, the recent CARE-HF trial supports improved mortality both from pump function and sudden death with CRT alone (without an implantable cardioverter-defibrillator [ICD]), and would argue against CRT augmenting arrhythmia susceptibility (27). Very little remains known about the molecular changes that are induced by chronic dyssynchrony and even less about what is reversed by CRT. However, ongoing studies using controlled animal models are addressing this intriguing question.
Trials and Metaanalyses of Cardiac Resynchronization Therapy
Since 2001, a series of clinical trials has been published that have demonstrated the efficacy of CRT in reducing both morbidity and mortality in patients with moderate to severe CHF
and LV dyssynchrony (Table 75.2). Two early studies demonstrating the feasibility of chronic multisite ventricular pacing to ameliorate HF symptoms were the MUSTIC (Multisite Stimulation in Cardiomyopathies) (28) and MIRACLE (Multicenter InSync Randomized Clinical Evaluation) (23) trials. In MUSTIC, the authors used a randomized, crossover approach to compare the effects of a 3-month period of atriobiventricular pacing versus 3 months without pacing. Study patients had class III HF despite optimal medical therapy, ejection fraction (EF) less than 35%, QRS greater than 150 ms, and LV dilation. The primary end point of the study was distance covered in the 6-minute walk test (6MWT); secondary end points included quality of life, peak 02 consumption during exercise, rates of death and hospitalization for CHF, and patient preference for either treatment period. In MUSTIC, CRT therapy resulted in a 23% increase in 6MWT distance and a 32% reduction (improvement) in the Minnesota quality-of-life score. Eighty-five percent of the patients preferred biventricular pacing. As such, MUSTIC was among the first trials to demonstrate that elective implantation of a multisite pacing system (or a mechanical device of any sort) could effectively treat HF symptoms in patients with moderate to severe disease.
and LV dyssynchrony (Table 75.2). Two early studies demonstrating the feasibility of chronic multisite ventricular pacing to ameliorate HF symptoms were the MUSTIC (Multisite Stimulation in Cardiomyopathies) (28) and MIRACLE (Multicenter InSync Randomized Clinical Evaluation) (23) trials. In MUSTIC, the authors used a randomized, crossover approach to compare the effects of a 3-month period of atriobiventricular pacing versus 3 months without pacing. Study patients had class III HF despite optimal medical therapy, ejection fraction (EF) less than 35%, QRS greater than 150 ms, and LV dilation. The primary end point of the study was distance covered in the 6-minute walk test (6MWT); secondary end points included quality of life, peak 02 consumption during exercise, rates of death and hospitalization for CHF, and patient preference for either treatment period. In MUSTIC, CRT therapy resulted in a 23% increase in 6MWT distance and a 32% reduction (improvement) in the Minnesota quality-of-life score. Eighty-five percent of the patients preferred biventricular pacing. As such, MUSTIC was among the first trials to demonstrate that elective implantation of a multisite pacing system (or a mechanical device of any sort) could effectively treat HF symptoms in patients with moderate to severe disease.
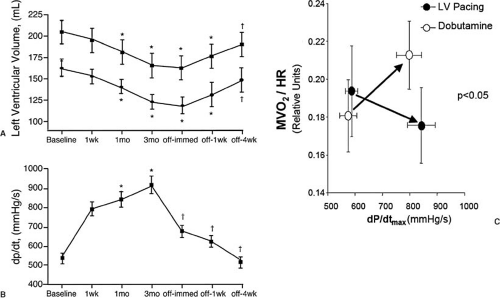 FIGURE 75.3. The effects of chronic CRT on (A) LV cavity volume and (B) dP/dtmax in dyssynchronous subjects (18). Cessation of CRT leads to an instantaneous decline in dP/dtmax, whereas LV cavity remodeling occurs more slowly, suggesting changes in tissue architecture rather than purely volume-mediated effects on cavity size. C: Mechanoenergetics in CRT and inotropic therapy with dobutamine, in which comparable increases in LV systolic function were achieved at substantially lower myocardial oxygen consumption rates (MVO2) in CRT compared with inotropic therapy (25). |
The MIRACLE trial investigated the effects of CRT in a larger, sicker, and possibly less dyssynchronous cohort of patients compared with those studied in MUSTIC. Patients in the MIRACLE trial had class III or class IV HF symptoms, EF less than 35%, ventricular dilation, and a QRS greater than 130 ms. Of 571 patients enrolled, 453 progressed to randomization in a prospective, double-blind investigation of 6 months of atriobiventricular pacing versus no pacing. Primary end points included distance walked in the 6MWT, quality of life, and New York Heart Association (NYHA) class; secondary end points included peak 02




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


