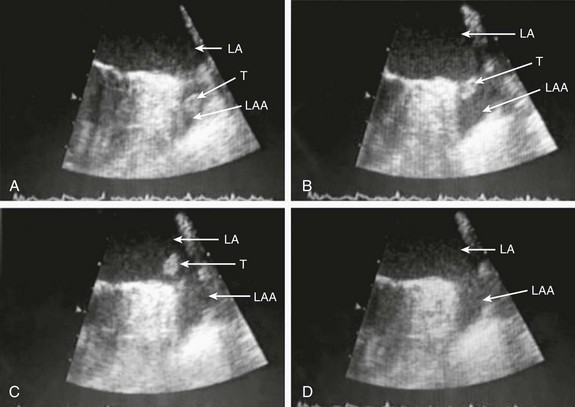
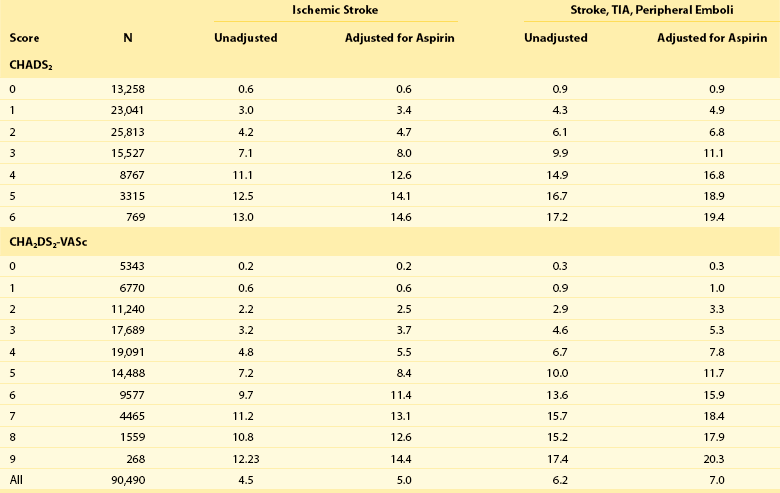
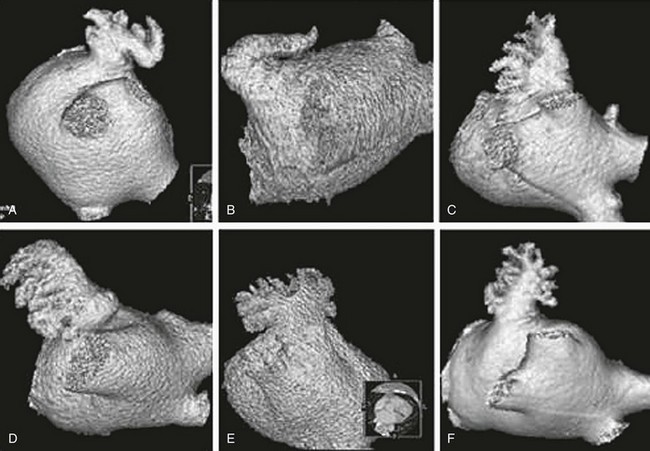
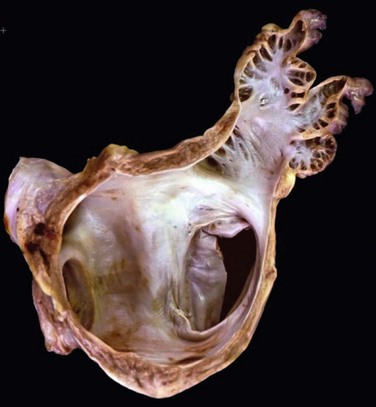
132 Occlusion of the left atrial appendage (LAA) has the potential for significant change in the strategies for stroke prevention in patients with nonvalvular atrial fibrillation (AF).1–4 These strategies are predicated on the putative mechanism of stroke in these patients.5 Multiple anecdotal cases6 (Figure 132-1) and a series of pathologic and echocardiographic studies have documented that the LAA is the nidus for thrombus, resulting in stroke in up to 90% of cases in this setting.5 The hypothesis of the link between thrombus in the LAA and subsequent stroke has been substantiated in the randomized PROTECT-AF trial,1,2 in which LAA occlusion alone was found to be noninferior to anticoagulation for stroke prevention. It must be remembered in this regard that other sources of thromboembolic stroke exist; for example, the left atrium (LA) itself in the setting of significant mitral valve and other structural heart disease, complicated patent foramen ovale, left ventricular thrombus, mobile aortic atheroma, and carotid arterial disease. In these later situations, chronic anticoagulant therapy can be effective in preventing stroke or systemic thromboembolism, but LAA occlusion by itself would have no beneficial effect. Figure 132-1 A, Echocardiographic assessment documenting thrombus (T) in the left atrial appendage (LAA). During the study, the thrombus is seen to migrate from the LAA out into the left atrium (LA) and then embolize from the LA (B-D). Given the well-known relationship between an increasing incidence of atrial fibrillation with advancing age, as well as the increase in incidence of stroke with advancing age, the numbers of patients at risk is estimated to increase dramatically.7–12 Between 10% and 30% of strokes occur in the setting of diagnosed or undiagnosed AF. Stroke prevention in the setting of AF has been the focus of intense investigation resulting in multiple professional societal guidelines10–12 and the evaluation and testing of risk scores.13–18 The most common risk score until recently was the CHADS2 score, which combined clinical factors that are useful in predicting the occurrence of stroke. This system has been superseded by CHADS2 VASc (Table 132-1). Table 132-1 Prediction of Stroke and Thromboembolism One of the largest studies evaluating risk stratification for both ischemic stroke and bleeding included 182,678 patients with atrial fibrillation in the Swedish National Registry.16 In a subset of 90,490 patients without warfarin throughout follow-up, the rate of stroke, transient ischemic attack, or peripheral embolization per 100 years at risk when adjusted for aspirin administration ranged from 0.9 to 19.4 using the CHADS2 score and 0.3 to 20.3 using the CHADS2DS2–VASc score (Table 132-2). There was improved predictive performance for the CHADS2DS2–VASc score for the composite thromboembolism endpoint. This expanded score appears to be of special importance in patients with lower CHADS2 scores of 0 to 1, and it is based on an incremental improvement in the predictive value of the added risk factors of age, sex, and the presence of vascular disease.17 In addition, it has important implications for identifying the need for anticoagulant therapy in these groups with lowest risk. Although other scoring systems have been developed,13 none of them have been widely used because of only having modest predictive value. Table 132-2 Stroke or Thromboembolism per 100 Patient-Years at Risk in Relation to CHADS2 and CHA2DS2-VASc Scores in 90,490 Patients Without Warfarin Throughout Follow-Up16 There has been controversy regarding the specific risk score that identifies a positive benefit or risk for anticoagulation; however, in general a CHADS2 score of 2 or greater is an indication,10,11 and in some documents 1 or greater is also strongly considered.12 It is important to remember that undertreatment with the occurrence of stroke is generally considered more harmful than overtreatment with potential bleeds. Conventional therapy has centered on warfarin,10,11 with which there were many problems, including absolute or relative contraindications, potential for bleeding, medication interactions, variability in dosing and effect, and the need for chronic intermittent monitoring. These issues resulted in the finding that warfarin was used in approximately 50% of patients in whom it was indicated; newer agents have been tested in large randomized trials involving in aggregate greater than 50,000 patients.19–29 In general, these new agents have been found to be more effective than warfarin with either somewhat less or similar bleeding risk, without the need for long-term monitoring of international normalized ratio (INR); adoption of these new agents remains highly variable. AF ablation, when successful, can allow the discontinuation of anticoagulation in low-risk patients. Nevertheless, convincing scientific data supporting its discontinuation in patients at moderate and high risk are not available. Current guidelines continue to mandate ongoing anticoagulation based on baseline stroke risk, regardless of the success of the ablation. 1. Some patients might have absolute or relative contraindications to both warfarin and the new anticoagulant agents. 2. The new agents might be associated with somewhat less bleeding, but the slope of the curve is only decreased and bleeding potential still increases over time. 3. The occurrence of gastrointestinal, pulmonary, and other side effects of new agents is not trivial. 4. In patients with higher CHADS2 or CHADS2 VASc scores, even after ablation, anticoagulation is recommended. 5. The life-long need for anticoagulants with the potential for side effects or drug-drug interactions and costs is substantial. 6. A substantial number of patients might develop coronary artery disease over time, requiring additional antiplatelet therapy and thus increasing the risk of bleeding known to accompany triple drug therapy. 1. It must be equally or more effective than alternative anticoagulation in clinical practice for stroke prevention, as demonstrated by large randomized clinical trials. The availability of data from well-executed, adequately powered randomized controlled trials is extremely important in this regard, although only one such trial has been published. 2. The risk-to-benefit ratio must be favorable. This issue is complex because, as with any invasive procedure, there will be at least some early procedural risks not present when only medication is prescribed; with the latter, there may be longer-term safety risks from hemorrhage. 3. The procedure must be performable in a substantial percentage of the patient candidates. 4. The procedure must be able available in a variety of institutions with well-trained specialists. 5. The approach should also be cost effective, without an adverse effect on quality of life. If these criteria can be met, LAA occlusion will play a substantial role for stroke prevention in patients with nonvalvular AF who are at increased risk of stroke. A variety of approaches for LAA occlusion has been developed and is being tested. An important initial consideration regarding feasibility relates to the detailed anatomy of the LAA itself,30–35 which is highly variable—often with multiple lobes and marked trabeculation. The orifice is usually asymmetrical with an oval or elliptical shape. This structure is universally located between the left upper pulmonary vein and the mitral annulus, but the three-dimensional spatial orientation of the body of the appendage is also variable. For example, its three-dimensional shape could be straight or have varying degrees of angulation or spiraling. Classification schemes of the orientation and location of the tip of the LAA have been developed and could have some application, although they are not widely used.34 Using computed tomographic imaging, Wang et al.35 identified specific features (Figure 132-2). Perhaps most importantly for device selection and use is either the presence of a marked bend in the proximal or middle portion of the dominant lobe or the redundant folding back of the LAA on itself. Such anatomical orientation can affect the ability to deploy a transseptal device. In patients without such an obvious bend, several different specific types were categorized depending on the number of lobes, the length of the dominant lobe, and the take-off of the lobes relative to the origin of the LAA. The angulation, length, and number of lobes therefore remain an extremely important consideration for the selection of potential closure approaches. Figure 132-2 Assessment of the shape of the left atrial appendage can affect selection of a specific device. Wang et al. have developed a classification scheme based on computed tomographic imaging. The “chicken wing” configuration (A, B) may be difficult to fully intubate and deliver a device. The other subtypes—windsock (C, D), califlower (E) and cactus (F)—can also affect the selection of specific devices.35 Another important consideration is fragility (Figure 132-3). The LAA has been described as “our most lethal human attachment.”36 The thickness of the LA wall itself varies substantially. Using 64-slice multidetector computed tomography, the average thickness of the LAA excluding fat was 1.89 ± 0.48 mm but ranged from 0.5 to 3.5 mm.37 Histologic assessment of the LAA itself documented small crevasses or pits or areas of wall thinning within the trabeculated appendage that could be transilluminated. Such paper-thin walls are highly vulnerable to manipulation or to the placement of anchors to maintain device position and prevent device displacement. Figure 132-3 Autopsy specimen of the left atrial appendage (LAA). The crypts within the pectinate muscles and the tip of the LAA are often extremely thin and possibly translucent.
Left Atrial Occluders/Isolation
Risk Factor
Points
CHADS2 score
Congestive Heart Failure
1
Hypertension
1
Age > 75 years
1
Diabetes
1
Prior stroke, TIA
2
CHA2DS2-VASc score
Congestive heart failure
1
Hypertension
1
Age ≥ 75 years
2
Diabetes
1
Stroke, TIA, TE
2
Vascular disease
1
Age 65 to 74 years
1
Female sex
1
Left Atrial Occluders/Isolation



