latrogenic Femoral Arteriovenous Fistulas
JASON P. JUNDT and TIMOTHY K. LIEM
Presentation
A 71-year-old female with hypertension, hyperlipidemia, atrial fibrillation on warfarin therapy, and a 50-pack-year smoking history presents for follow-up 2 weeks after an aortogram with bilateral lower extremity runoff. The procedure was performed for right lower extremity claudication symptoms. Noninvasive studies demonstrated ankle-brachial indices (ABIs) of 0.68 on the right and 0.81 on the left. The procedure was performed via left femoral access with anticoagulation managed by low molecular weight heparin bridge. The patient’s vital signs are normal, but she complains of worsening left leg claudication symptoms since the procedure. There are no groin hematomas but mild swelling in the location of the left femoral puncture. Distal signals are present but biphasic. Auscultation demonstrates a bruit over the left common femoral artery.
Differential Diagnosis
Complications from femoral artery access are common, occurring in 1% to 14% of procedures. The complications are diverse and include hematoma (10%), pseudoaneurysm (2% to 8%), active bleeding (2.5%), arteriovenous fistula (AVF) (0.1%), thrombosis (less than 0.1%), embolism (less than 0.1%), and retroperitoneal hematoma (less than 0.1%). While the majority of these complications are minor and do not require intervention, some are severe and insidious and occasionally require urgent operative intervention (e.g., hemorrhage into the retroperitoneum).
A history and physical examination consistent with worsening claudication in conjunction with swelling and bruit are suspicious for an iatrogenic AVF. Furthermore, this patient has risk factors for AVF formation following percutaneous intervention, such as female gender (OR = 1.84), arterial hypertension (OR = 1.86), warfarin therapy (OR = 2.34), and left-sided puncture (OR = 2.21). An additional risk factor, not present in our patient, includes intraprocedural heparin dosage of greater than 12,500 units.
Workup
The patient undergoes further evaluation with an arterial duplex examination. Using an 8- to 12-MHz probe and color flow pulsed Doppler duplex ultrasound, an iatrogenic AVF is diagnosed (Fig. 1) between the profunda femoris artery and the femoral vein. A less than 0.2-cm communicating channel is visualized, and no pseudoaneurysm is seen. Laboratory findings are unremarkable.
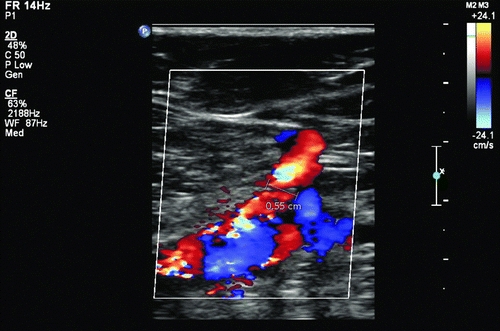
FIGURE 1 Iatrogenic arteriovenous fistula between the deep femoral artery and the femoral vein.
Diagnosis and Treatment
Arteriovenous fistulas and pseudoaneurysms of the lower extremity arteries are most commonly iatrogenic or secondary to traumatic injury. Over the past 20 years, percutaneous interventions have exponentially increased, making iatrogenic AVFs a more frequently encountered clinical entity. A history of prior intervention in the setting of worsening claudication, nonhealing ulcers, and symptoms of congestive heart failure are suspicious for an iatrogenic AVF. Physical examination can demonstrate stigmata of previous groin access, swelling, bruising, erythema, and a palpable thrill. Auscultation over the previous access point will demonstrate a continuous machinery type bruit, and occasionally, temporary occlusion of large AVFs will cause a reflexive slowing of the heart rate (Nicoladoni-Branham sign).
Imaging studies including duplex ultrasound with pulsed Doppler and color flow imaging (DUS), computerized tomography angiogram (CTA), and conventional angiogram (CA) all can confirm the diagnosis and define the anatomy. Specific findings associated with AVFs on DUS include the presence of flow jets, and conversion of the typical high-resistance arterial waveform to a lower-resistance pattern with persistent diastolic flow (Fig. 2). In addition, some AVFs demonstrate a “visual bruit” (color mixing outside of the vessel wall), caused by turbulence-associated soft tissue vibration (Fig. 3). Computerized tomography typically will demonstrate early opacification of the vena cava during the arterial phase, with asymmetric enhancement and distention of venous structures due to venous hypertension ipsilateral and distal to the AVF. Conventional angiography will demonstrate the location, neck if present, and flow jet with characteristic rapid venous filling through the AVF. It can be useful both for diagnosis and concurrent treatment (Fig. 4).
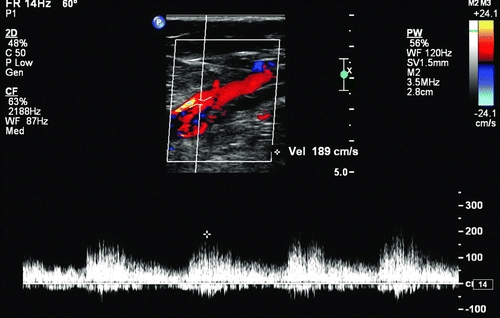
FIGURE 2 Doppler-derived waveforms in a femoral artery, demonstrating low-resistance flow caused by an arteriovenous fistula.
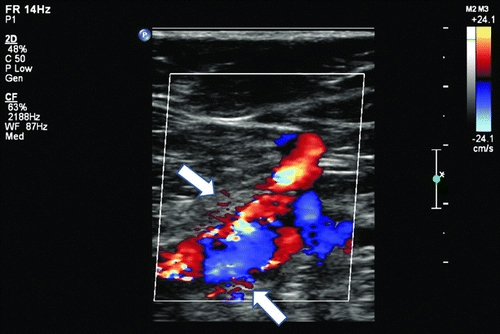
FIGURE 3 Soft tissue vibration caused by an arteriovenous fistula may cause a “visual bruit” or flow artifacts outside of the vessel wall.
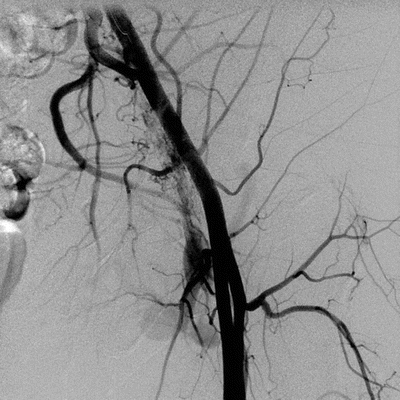
FIGURE 4 Conventional angiography demonstrating early venous filling associated with an iatrogenic arteriovenous fistula.
Surgical treatment for iatrogenic AVFs seldom is necessary, since the spontaneous closure rate at 1-year follow-up is greater than 80% with nonoperative management. Of the iatrogenic AVFs that close at 1 year, nearly 70% will close within the first 4 months, with the remainder closing within 12 months. Surgical or catheter-based treatment should be considered in patients with symptoms such as severe pain and edema, and in the unusual patient with worsening congestive heart failure.
There are three treatment options for iatrogenic AVFs: ultrasound-guided compression repair (UGCR), endovascular exclusion (either with a covered stent graft or vascular plug in the communicating channel), or open repair. Although UGCR is the least invasive option, it is typically poorly tolerated, and given the nature of iatrogenic AVFs (short necks and high flow), it has the lowest rate of treatment success. Endovascular therapy, consisting of covered stent placement or embolization with a vascular plug, is becoming utilized more commonly with numerous case reports and series demonstrating both safety and efficacy. Open surgical repair has a very high technical success rate, but adverse event rates as high as 20% have been reported, mostly in relation to groin wounds complications.
Endovascular and Surgical Approach
If noninvasive attempts to close symptomatic iatrogenic AVFs are not successful, either endovascular or open intervention is indicated. Patients with a hostile groin (due to multiple prior surgeries), those who are deemed to be at high risk for infection or wound complications (large pannus), and unstable patients (recall that many of these patients underwent coronary angiogram and stenting due to myocardial infarction) may benefit from a minimally invasive approach, either with embolization or placement of a covered stent. Embolization is more suitable for chronic AVFs that have well-developed tortuous fistulous tracts and for distal or deep femoral fistulas that may be the result of blunt or penetrating trauma. Stent graft coverage is more suitable for patients with large artery fistulas (common femoral or superficial femoral), especially when the fistulous tract may be short.
Endovascular repair of a femoral AVF often is approached with a retrograde crossover technique from the contralateral groin, since antegrade femoral puncture results in inadequate working lengths. Access is obtained using DUS or fluoroscopic guidance as needed and a 4-French or 5-French micropuncture set. A Benson wire is advanced into the distal aorta. A 4-French or 5-French pigtail catheter is then placed in the distal aorta, with aortography perform to delineate the iliofemoral anatomy as well as the iatrogenic AVF. At this point, measurements are obtained to plan for stent or Amplatzer selection as well as sheath size for delivery. The aortic bifurcation is navigated using a RIM catheter, Sos Omni, or other appropriate catheter. A J-wire or glidewire can then be advanced down the contralateral common iliac, external iliac, and common femoral arteries.
In anticipation of sheath placement, the patient is anticoagulated with 80 U/kg of heparin sulfate. A long precurved sheath, with a diameter appropriate for deployment of the selected stent, is advanced over the guidewire and stationed proximal to the AVF. An angiogram is performed through the sheath, and SmartMask or roadmap setting is used to precisely place a covered stent across the abnormal communication. Completion angiogram is performed to confirm sealing of the fistula. The patient receives a follow-up duplex evaluation and typically is discharged home on the same day. Postoperative medications include 3 months of dual antiplatelet therapy followed by indefinite aspirin.
Patients who are a good operative risk, those with unsuitable endovascular anatomy, and those who have failed endovascular repair may undergo open surgical correction. Open surgical repair usually is performed under general anesthesia. A longitudinal or oblique incision is made directly over the arterial segment of the iatrogenic AVF. The skin and subcutaneous tissue are divided using electrocautery with proximal and distal control of both venous and arterial structures. Longer fistulous tracts usually can be divided without clamping the adjacent femoral artery or vein. If the artery is injured or if the fistula closure results in significant arterial or venous stenosis, then heparin should be administered and patch angioplasty performed. Continuous wave Doppler should then been used to confirm venous patency and conversion of the arterial waveform into a typical higher-resistance signal (Table 1).
TABLE 1. Key Technical Steps and Potential Pitfalls




