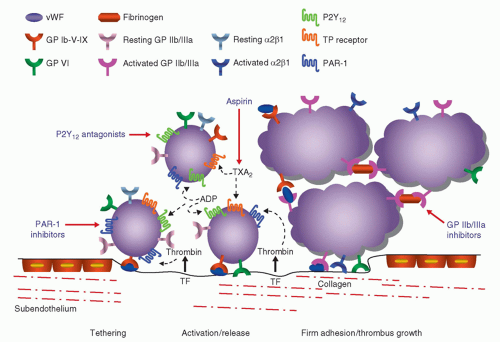
Study |
N |
Study Drugs |
Setting |
Primary Endpoint |
Resultsa |
CURE |
12,562 |
Aspirin + clopidogrelbvs. aspirin |
UA/NSTEMI |
Cardiovascular death, nonfatal MI, or stroke at 1 year |
9.3% vs. 11.4%; RR = 0.80 (0.72-0.90) |
PCI-CURE |
2,658 |
Aspirin + clopidogrelbvs. aspirin |
PCI patients from CURE |
Cardiovascular death, MI, or urgent TVR at 30 days |
4.5% vs. 6.4%; RR = 0.70 (0.50-0.97) |
CREDO |
2,116 |
Aspirin + clopidogrelbvs. aspirin |
Elective PCI |
Death, MI, or stroke at 1 year |
8.5% vs. 11.5%; RRR = 26.9% (3.9%-44.4%) |
COMMIT |
45,852 |
Aspirin + clopidogrelbvs. aspirin |
Acute MI (93% STEMI) |
Death, reinfarction, or stroke at discharge or at 28 days |
9.2% vs. 10.1%; OR = 0.91 (0.86-0.97) |
CLARITY |
3,491 |
Aspirin + clopidogrelbvs. aspirin |
STEMI with fibrinolysis |
Occluded infarct-related artery on angiography, death, or recurrent MI before angiography |
15.0% vs. 21.7%; OR = 0.64 (0.53-0.76) |
PCI-CLARITY |
1,863 |
Aspirin + clopidogrelbvs. aspirin |
PCI patients from CLARITY |
Cardiovascular death, recurrent MI, or stroke at 30 days |
3.6% vs. 6.2%; OR = 0.54 (0.35-0.85) |
CURRENT-OASIS 7 |
25,086 |
Aspirin + double-dose clopidogrelbvs. aspirin + standard-dose clopidogrelb |
ACS patients referred for invasive strategy |
Cardiovascular death, MI, or stroke at 30 days |
4.2% vs. 4.4%; HR = 0.94 (0.83-1.06) |
CURRENT-OASIS 7 (PCI cohort) |
17,263 |
Aspirin + double-dose clopidogrelbvs. aspirin + standard-dose clopidogrel |
PCI patients from CURRENT-OASIS 7 |
Cardiovascular death, MI, or stroke at 30 days |
3.9% vs. 4.5%; HR = 0.86 (0.74-0.99) |
TRITON-TIMI 38 |
13,608 |
Aspirin + prasugrel vs. aspirin + clopidogrelb |
ACS patients undergoing PCI |
Cardiovascular death, nonfatal MI, or nonfatal stroke up to 15 months |
9.9% vs. 12.1%; HR = 0.81 (0.73-0.90) |
PLATO |
18,624 |
Aspirin + ticagrelor vs. aspirin + clopidogrelb |
ACS patients |
Death from vascular causes, MI, or stroke at 12 months |
9.8% vs. 11.7%; HR = 0.84 (0.77-0.92) |
PLATO (invasive) |
13,108 |
Aspirin + ticagrelor vs. aspirin + clopidogrelb |
PCI patients from PLATO |
Death from vascular causes, MI, or stroke at 12 months |
8.9% vs. 10.6%; HR = 0.84 (0.75-0.94) |
ACS, acute coronary syndrome; MI, myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; RR, relative risk; RRR, relative risk reduction; STEMI, ST-elevation myocardial infarction; TVR, target vessel revascularization; UA, unstable angina; CURE, Clopidogrel in Unstable Angina to Prevent Recurrent Events trial; CREDO, Clopidogrel for the Reduction of Events During Observation trial; COMMIT, Clopidogrel and Metoprolol in Myocardial Infarction trial; CLARITY, Clopidogrel as Adjunctive Reperfusion Therapy trial; CURRENT-OASIS-7, Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Optimal Antiplatelet Strategy for Intervention trial; TRITON, Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel; PLATO, Platelet Inhibition and Outcomes. |
a Results are expressed as percentage of events and association measure (95% confidence interval).
b Clopidogrel was given as a loading dose of 300 mg and then 75 mg daily in CURE, CREDO, CLARITY, and COMMIT. In CURRENT/OASIS 7, double-dose clopidogrel was defined as a 600-mg loading dose and 150 mg once daily for 7 days, followed by 75 mg once daily; standard-dose clopidogrel was defined as a 300-mg loading dose, followed by 75 mg once daily. Patients were also randomized to receive low-dose (75-100 mg/day) or high-dose (300-325 mg/day) aspirin. Prasugrel was given as a 60-mg loading dose, followed by 10 mg once daily. Ticagrelor was given as a 180-mg loading dose, followed by 90 mg twice daily. |