INDICATIONS/CONTRAINDICATIONS
Chronic or recurrent GERD with breakthrough symptoms on medical therapy is the most common indication for LNF. LNF should only be considered in patients with documented evidence of pathologic GERD.
Objective evidence of GERD is given as follows:
 Reflux esophagitis–endoscopic/pathologic
Reflux esophagitis–endoscopic/pathologic
 Peptic stricture
Peptic stricture
 Barrett’s esophagus (BE)
Barrett’s esophagus (BE)
 Abnormal 24-hour pH score
Abnormal 24-hour pH score
 Abnormal impedance pH score
Abnormal impedance pH score
In patients with typical symptoms like heartburn and regurgitation, especially with good relief with medical therapy, at least one type of objective evidence of reflux should be sought before proceeding with surgery. In patients with atypical GERD symptoms, a minimum of two types of objective evidence of GERD should be sought, in addition to ruling out other causes, before LNF is offered to the patient. The best indicator of success of LNF is a good response to medical therapy.
Indications for LNF are as follows:
 Breakthrough symptoms of heartburn and regurgitation on maximal medical therapy.
Breakthrough symptoms of heartburn and regurgitation on maximal medical therapy.
 Respiratory symptoms attributable to pathologic GERD.
Respiratory symptoms attributable to pathologic GERD.
 Laryngopharyngeal reflux symptoms with documented pathologic GERD.
Laryngopharyngeal reflux symptoms with documented pathologic GERD.
 Volume regurgitation associated with nocturnal symptoms.
Volume regurgitation associated with nocturnal symptoms.
 Drug-dependent patients with documented abnormal 24-hour pH score especially with a mechanically defective LES.
Drug-dependent patients with documented abnormal 24-hour pH score especially with a mechanically defective LES.
 Evidence of esophageal damage: Stricture, reflux esophagitis, or BE.
Evidence of esophageal damage: Stricture, reflux esophagitis, or BE.
LNF is contraindicated in patients with severely impaired esophageal motility or scleroderma. In patients with severely delayed gastric emptying and a competent LES/no HH, a gastric drainage procedure rather than LNF should be considered. In patients with both delayed gastric emptying and pathologic GERD, an LNF with distal gastrectomy and Roux-en-Y (RNY) reconstruction should be planned. The presence of high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) is an absolute contraindication for LNF. In patients with a history of HGD and/or superficial adenocarcinoma that has been documented to regress with endoscopic ablative or resection therapy, LNF can be carefully considered. There is uncertain evidence that LNF significantly decreases the progression of BE to EAC, and LNF should not be done for that reason alone. Patients with undilatable peptic stricture or short esophagus are better served with esophageal resection or Collis gastroplasty with fundoplication respectively, rather than LNF.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
In addition to general preoperative evaluation to assess patient fitness to undergo surgery, a detailed assessment should be done. History of symptoms and response to medical therapy should be carefully documented. It is important to remember that patient-perceived symptoms can be due to different etiologies. For example, it is common for a patient with achalasia to be treated medically for GERD prior to correct diagnosis. In such situations though, delay in diagnosis is erroneous, but mere institution of medical therapy would not be harmful, while proceeding with fundoplication would be disastrous. Hence it is imperative that the operating surgeon plays an active role in establishing diagnosis and completely understanding individual patient pathophysiology. As mentioned previously, surgery should only be undertaken after the objective documentation of GERD in an appropriate clinical setting.
 Esophagogastroduodenoscopy (EGD): An upper endoscopy should always be performed prior to surgical intervention. This allows for direct assessment of esophageal mucosa and cardia competence/HH and histologic diagnosis of BE (if present).
Esophagogastroduodenoscopy (EGD): An upper endoscopy should always be performed prior to surgical intervention. This allows for direct assessment of esophageal mucosa and cardia competence/HH and histologic diagnosis of BE (if present).
 Contrast study: An upright and supine barium esophagram delineates the size and type of HH. We routinely use both liquid and solid material to help assess esophageal motility. The 13-mm tablet helps reveal subtle strictures that might be missed on endoscopy.
Contrast study: An upright and supine barium esophagram delineates the size and type of HH. We routinely use both liquid and solid material to help assess esophageal motility. The 13-mm tablet helps reveal subtle strictures that might be missed on endoscopy.
 Esophageal manometry: Esophageal motility helps determine the type of fundoplication that should be performed. Though there is no evidence to support a tailored fundoplication based on esophageal motility, most surgeons in the United States proceed with partial fundoplication in patients with ineffective esophageal motility. Introduction of high-resolution manometry holds the promise to better delineate motility disorders and aid the surgical decision process.
Esophageal manometry: Esophageal motility helps determine the type of fundoplication that should be performed. Though there is no evidence to support a tailored fundoplication based on esophageal motility, most surgeons in the United States proceed with partial fundoplication in patients with ineffective esophageal motility. Introduction of high-resolution manometry holds the promise to better delineate motility disorders and aid the surgical decision process.
 24-hour pH: Prolonged distal esophageal acid exposure is the gold standard for objective assessment for GERD. Impedance pH is used to document nonacidic reflux and, in my opinion, is of real use only in very select patients. One must note that distal esophageal pH changes are a marker of reflux, not the disease itself (which is reflux of gastric contents). Dual pH monitoring is used to document proximal esophageal acid exposure in patients with extraesophageal symptoms.
24-hour pH: Prolonged distal esophageal acid exposure is the gold standard for objective assessment for GERD. Impedance pH is used to document nonacidic reflux and, in my opinion, is of real use only in very select patients. One must note that distal esophageal pH changes are a marker of reflux, not the disease itself (which is reflux of gastric contents). Dual pH monitoring is used to document proximal esophageal acid exposure in patients with extraesophageal symptoms.
 Wireless (Bravo) 48-hour pH: Recently, wireless pH monitoring using a radiotelemetric capsule, attached to the wall of the esophagus (Bravo pH monitoring system; Medtronic, Minneapolis, MN), has become widespread. The Bravo capsule allows prolonged monitoring and is better tolerated by patients than pH probes placed on a transnasal catheter. Although the Bravo probe does not assess impedance pH (nonacidic reflux), it promotes a more typical diet and daily activities during the monitoring period, and is a useful tool for assessing esophageal acid exposure.
Wireless (Bravo) 48-hour pH: Recently, wireless pH monitoring using a radiotelemetric capsule, attached to the wall of the esophagus (Bravo pH monitoring system; Medtronic, Minneapolis, MN), has become widespread. The Bravo capsule allows prolonged monitoring and is better tolerated by patients than pH probes placed on a transnasal catheter. Although the Bravo probe does not assess impedance pH (nonacidic reflux), it promotes a more typical diet and daily activities during the monitoring period, and is a useful tool for assessing esophageal acid exposure.
 Gastric emptying study: A nuclear medicine gastric emptying study should be done in patients with significant bloating and also in patients with pathologic GERD if they appear to have a competent LES and no HH. These results should be interpreted cautiously as there may be poor symptom correlation.
Gastric emptying study: A nuclear medicine gastric emptying study should be done in patients with significant bloating and also in patients with pathologic GERD if they appear to have a competent LES and no HH. These results should be interpreted cautiously as there may be poor symptom correlation.
 SURGERY
SURGERY
The goal of surgery is to create a tension-free infradiaphragmatic fundoplication over the distal esophagus. This recreates a 2 to 3 cm intra-abdominal length of the esophagus and places the high-pressure gastric fundus around the gastroesophageal junction (GEJ), restoring a competent LES complex.
Patients with documented delayed gastric emptying are asked to be on a clear liquid diet for 2 to 3 days before surgery. Antiplatelet agents and anticoagulants are withheld appropriately. A single dose of a first generation cephalosporin (cefazolin, 1 g) is given within 30 minutes of incision. Deep vein thrombosis (DVT) prophylaxis is administered with 5000 U subcutaneous heparin and lower extremity sequential compressive devices. Surgery is done under general anesthesia and specific precautions should be paid to prevent aspiration during induction. We routinely give ondansetron iv 30 minutes before finishing the case. In patients with history of postoperative nausea, we have low threshold to use propofol drip and give dexamethasone iv at induction.
Room Setup
Use of dedicated minimally invasive operating room suite is helpful. We use three overhead hanging monitors near the head of the bed. The surgeon stands between the legs of the patient, first assistant to his/her right (left side of the patient), and the camera holder on the left (right side of the patient). After the start of the case, the instrument Mayo stand is placed from the left of the patient over the chest. The instrument table is on the left near the foot-end. The instrument cords are over the right shoulder of the patient. In addition, endoscopic equipment should be available for assessment (Fig. 1.1).
Positioning
The patient is positioned in an inverted-Y (modified lithotomy) position. An operating bed with a great deal of vertical range and degree of incline capacity should be used. We use the AlphaMaxx bed (Maquet Getinge AB, Rastatt, Germany). Legs are on split extensions with footboards. Both arms are tucked in; if there is a need to extend one or both arms, the arm boards should be positioned to prevent hyperextension at the shoulders (one must keep in mind that the patient may slide downward when the reverse Trendelenburg position is used during the case). The patient is prepped, draped from mid-chest down to the pubis symphysis (Fig. 1.2). If there is a high degree of suspicion for short esophagus, the left chest is also prepped in the field as we use a left thoracoscopic Collis gastroplasty.
Peritoneal Access
Peritoneal access is obtained as per surgeon’s preference. We use a Veress step needle to obtain pneumoperitoneum through a 5-mm skin incision made just to the left of midline, a third of the way up between the umbilicus and the xiphoid. An Optiview 5-mm cannula (Endopath Ethicon Endosurgery, Cincinnati, OH) is inserted using a zero-degree 5-mm laparoscope. Alternatively, an open technique using a Hasson cannula can be used. A pneumoperitoneum of 12 to 15 mm Hg is achieved. After initial diagnostic laparoscopy, the table is slowly positioned in a steep reverse Trendelenburg position. The patient’s blood pressure should be closely monitored as increased intra-abdominal pressure and positioning significantly decrease cardiac venous return and can affect homodynamic status. In such a situation, pneumoperitoneum is evacuated and the table is flattened. Resuming abdominal insufflation and repositioning the table more slowly after a fluid bolus is usually uneventful. Further, cannulae are inserted under laparoscopic guidance.
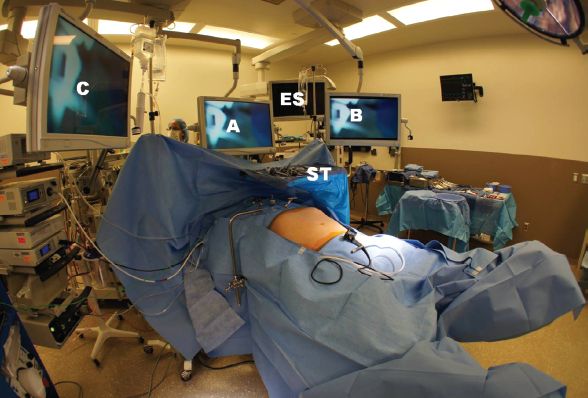
Figure 1.1 This shows the room set up. Scrub table (ST) comes across the patient’s chest from the left side. Monitor (A) is over the head of the bed; monitor (B) and (C) on the left and right of the patient near the head of the bed are adjusted for use by the camera holder and the first assistant, respectively. The endoscopy system (ES) is available to be placed from the left near the anesthesiologist. Patient is positioned in inverted-Y position.
Remaining Cannula and Liver Retractor
A 5-mm cannula is placed one to two fingerbreadth below the costal margin in the left anterior axillary line; this is for the assistant’s instruments. A 5-mm incision is made and the fascial opening created with a trocar just to the left of the xiphoid. Through this, a Nathanson liver retractor is inserted and positioned to retract the left lobe of liver superiorly. The retractor is attached to a table-mounted system using the Iron Intern (Automated Medical Products, Edison, NJ). Another 5-mm cannula is placed (using the surgeon’s left hand) 4 cm to the right of midline between the camera port and the xiphoid. It is directed toward the hiatus, and may traverse the falciform ligament. Finally we use a 12-mm cannula in the left upper quadrant below the costal cartilage in the midclavicular line. Using a 12-mm cannula allows the use of needles without “skiing,” though some surgeons use all 5-mm trocars (Fig. 1.3). Different liver retractors are available and can be used depending on surgeon preference and familiarity. Many different cannula placements have been described and the surgeon should use the one they feel most comfortable with.
Figure 1.2 Surgical field extends from above the xiphoid to the pubic symphysis and from the mid axillary line on each side. The surgeon stands between the patient’s legs.



