Laboratory Investigations in the Diagnosis of Pulmonary Diseases
Philip G. Robinson
This chapter introduces the diagnostic techniques available from the pathology laboratory for the diagnosis of pulmonary diseases. It is divided into two sections, one on the anatomic laboratory and the other on the clinical laboratory. The anatomic section deals with surgical pathology and cytology specimens, including the different types of specimens, how they should be handled, and different examining techniques. The clinical section deals with infectious agents and the laboratory techniques available to detect them. Leslie and Wick77 reviewed the optimal processing of lung specimens for histopathology and microbiology.
Anatomic Specimens
The different types of anatomic specimens are listed in Table 15-1. They range from diagnostic biopsies to specimens resected for therapeutic purposes. The types of specimens vary from pleural biopsies to pneumonectomies; they vary in size from several millimeters to an entire lung. A multidisciplinary approach between the surgeon, radiologist, pathologist, and pulmonologist will assure the appropriate diagnostic biopsy or treatment for the patient.
Approach to Lung Biopsies
Lung biopsies are done for a variety of problems including tumors, interstitial lung diseases, and pulmonary infiltrates. The methods of obtaining specimens and the types of specimens are varied. Depending on the patient’s clinical course, the suspected diagnosis, and the location of the lesion, the endobronchial or transbronchial biopsy is the procedure of choice because of its low morbidity and cost. Gal46 (2005) describes the transbronchial biopsy as being performed in order to avoid either an open-lung biopsy or a video-assisted thoracoscopic surgery (VATS) biopsy. The transbronchial biopsy has varied reliability depending on the type of disease (Table 15-2). Its main uses are to diagnose lung cancer and opportunistic infections in the immunocompromised as well as to monitor lung transplants. It is unreliable for the diagnosis of interstitial lung diseases. It may be used in combination with bronchoalveolar lavage (BAL) or bronchial brushings, which produce cytologic specimens. If the lesion is a solitary nodule and is out of reach of a bronchoscope, the transthoracic fine-needle aspiration or needle biopsy would be the next procedure of choice. The complications from bronchoscopy and needle biopsy or aspiration most frequently include pneumothorax or hemorrhage.
Halkos and colleagues,53 in their review, point out that the lung biopsy is the most sensitive and specific way to obtain a diagnosis of the interstitial lung diseases. Further, Travis and colleagues138 recommend that in absence of contraindications a lung biopsy is the best way to arrive at the appropriate diagnosis. In an immunosuppressed patient, the first procedure would be a bronchoscopy with biopsy and a BAL. Should these procedures fail to yield a diagnosis, the next step would be an open-lung
biopsy or a VATS biopsy. Jain and associates63 found that in immunocompromised patients, transbronchial biopsy and BAL were synergistic in that they had a greater diagnostic yield when used together rather than individually. Should these procedures not yield a diagnosis, an open-lung biopsy would be indicated. Kao and coworkers67 used open-lung biopsy in patients with early-stage acute respiratory distress syndrome (ARDS). They made specific diagnoses in 18 of 41 patients (44%). Further, the biopsies led to treatment alterations in 30 of the 41 patients (73%). Malhotra and Patel80 commented on the findings and pointed out that the prognosis for patients with ARDS is poor despite our increased knowledge of this entity.
biopsy or a VATS biopsy. Jain and associates63 found that in immunocompromised patients, transbronchial biopsy and BAL were synergistic in that they had a greater diagnostic yield when used together rather than individually. Should these procedures not yield a diagnosis, an open-lung biopsy would be indicated. Kao and coworkers67 used open-lung biopsy in patients with early-stage acute respiratory distress syndrome (ARDS). They made specific diagnoses in 18 of 41 patients (44%). Further, the biopsies led to treatment alterations in 30 of the 41 patients (73%). Malhotra and Patel80 commented on the findings and pointed out that the prognosis for patients with ARDS is poor despite our increased knowledge of this entity.
Table 15-1 Types of Surgical Pathology and Cytology Specimens | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 15-2 Reliability of Transbronchial Biopsy With Regard to Various Diseases | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Steinberg and associates123 found open-lung biopsies to be an important tool in pediatric patients. Twenty-six children underwent biopsy, and the biopsies were diagnostic in 25 of these (96%). Therapeutic changes were instituted in 18 patients (69%) based on the results of the biopsy. In another study, Choong and colleagues22 reviewed the open-lung biopsies of 89 of 224 patients who had undergone lung or heart–lung transplants. The indications for biopsy were for suspicion of bronchiolitis obliterans (70 patients), posttransplantation lymphoproliferative disorder (15 patients), infection (8 patients), and unexplained respiratory failure (10 patients). A new diagnosis was made on 49 biopsies (48%), the suspected diagnosis was confirmed in 50 biopsies (49%), and 4 biopsies (3%) were nondiagnostic. A change of therapy occurred in 69% of the cases. There was no mortality as a direct result of the open-lung biopsy, but 11 major and 22 minor complications occurred in 103 procedures. White and associates147 evaluated the results of open-lung biopsy in 63 patients with hematologic malignancies and unknown pulmonary processes who underwent 67 biopsies. A specific diagnosis was established in 41 (62%) of the biopsies and changes in therapy were made in 37 (57%) of patients. A focal radiographic abnormality rather than a diffuse one was more predictive of a specific diagnosis (79% as opposed to 36%). The specific diagnoses were inflammatory (23%), infectious (21%), and malignant (18%) disorders. The most common inflammatory disorder was bronchiolitis obliterans organizing pneumonia, and the most common infections were bacterial and fungal.
Pleural Biopsy
The thoracic cavity contains two pleural surfaces. The visceral pleura covers the lung and the parietal pleura lines the thoracic cavity. Pleural biopsies come from the parietal pleura. They range in size from 1 to 5 mm and are submitted in formalin. Maskell and associates82 found that CT-guided pleural biopsies were better than random biopsies in diagnosing malignancies where the pleural effusion was cytologically negative. They found that one biopsy pass was sufficient if the gross examination revealed enough tissue. They diagnosed malignancy in 87% of the CT-guided biopsies as opposed to 44% in the random biopsy group. Routine pleural biopsies are useful for evaluating pleural inflammation and infections, particularly tuberculosis. Christopher and colleagues23 performed pleural biopsies in 27 patients with exudative effusions. The pleural biopsy showed tuberculosis in 12 (75%) of the 16 patients with the disease. In patients with malignancies, the pleural biopsy showed the disease in 5 (71%) of the 7 patients. Jimenez and associates65 tried to determine the optimal number of pleural biopsies needed to establish a diagnosis. They found that with the carcinoma group, the more random biopsies performed, the greater the chance of diagnosing carcinoma. With one biopsy, 54% of the patients could be diagnosed. With four biopsies, that percentage rose to 89%. In contrast, the percentage did not increase with additional biopsies in the patients who had tuberculosis. Pleural biopsies may be useful in evaluating patients with pleural disease.
Endobronchial and Transbronchial Biopsy
Transbronchial or endobronchial biopsies are usually obtained through a fiberoptic bronchoscope, although they may be obtained through a rigid bronchoscope. Since the introduction of the fiberoptic bronchoscope, the use of the rigid bronchoscope has declined. Endobronchial or transbronchial biopsy specimens are composed of four to five pieces of tissue that are submitted in an appropriate fixative, usually formalin, with each piece measuring 1 to 2 mm in greatest dimension. Serial sections are performed on the block because the lesion may not be present on the first slide. Unstained sections may be made when the block is first cut, because owing to the small size of the specimen, it is technically difficult to go back and make additional sections. The unstained sections may be used for special stains to diagnose infections, tumors, or other processes.
Yoshikawa and colleagues157 described the value of using ultrasound and a peripheral guide sheath for the transbronchial biopsy of peripheral pulmonary nodules. They investigated 123 peripheral pulmonary nodules in 121 patients. They inserted and advanced the endobronchial ultrasound with a guide sheath toward the lesion. Once they had an ultrasound image, they performed a biopsy. They were able to diagnose 61 of the 123 peripheral nodules (61.8%) by this technique. They found that the lesions larger than 20 mm had a greater diagnostic yield than those under 20 mm. The location was also important with lesions in the lingula and right middle lobe having a greater yield than lesions in other locations. Finally, solid lesions had a greater diagnostic yield than did nonsolid lesions. Ernst and associates40 reviewed the technique and the technology behind it. Aboyoun and colleagues4 point out the value of transbronchial biopsy in following lung rejection after lung transplant.
Lung Needle Biopsy
Lung needle biopsies and aspirations are obtained under radiographic computed tomography (CT) guidance and are directed at a specific lesion. The techniques for obtaining lung biopsies and aspirations are very similar; the basic technique is described further on. Klein and Zarka73 reviewed the use of the transthoracic needle biopsy, including the indications, contraindications, imaging modalities, and biopsy techniques. The most common indication is to evaluate a solitary pulmonary nodule; other indications are to confirm metastatic disease to the lung, diagnose a mediastinal mass, diagnose pleural thickening, sample possible infectious nodules for cultures, and stage lung cancer. The contraindications are a bleeding diathesis, uncooperative patient, contralateral pneumonectomy, severe emphysema, and a vascular structure in the pathway of the needle.
Most biopsies are performed under CT guidance. The CT images allow the radiologist to place the needle in the tumor module. The coaxial needle placement technique is commonly used. A guide needle of about 19 gauge is inserted into the lesion. The stylet is removed, and either a cutting needle or an aspiration needle is placed into the lesion. A common cutting needle is a spring-loaded one with either an end-cut or side-notched device (e.g., Biopty [Bard Reynosa, S.S. de C.V., Reynosa, Mexico], ASAP-18 [Meditech, Boston Scientific Group, Natick, MA], Temno bone needle [Bauer Medical, Clearwater, FL]). Once the biopsy needle is in the lesion, it is fired. Gazelle and Haaga48 reviewed the characteristics of the various needles.
The needle biopsies usually produce a specimen that consists of between one and three cylindrical fragments of tissue that measure about 10 mm in length by 1 mm in diameter. Like transbronchial biopsies, needle biopsies are so small that additional sections are usually cut when the slides are first made. The first slide should be stained with hematoxylin and eosin (H&E). After reviewing that slide and gaining an impression of the pathologic process, the pathologist can determine what stains to perform on the remaining slides. The immuno- histochemical technique is described in greater detail in Chapter 168.
Fielding and coworkers42 compared endobronchial ultrasound guide-sheath biopsy to a CT guided fine-needle aspiration or biopsy. They found that in 140 cases with a mean lesion size of 29 mm, the overall diagnostic sensitivity was 66%. In comparison, for those lesions that did not touch the visceral pleura, the diagnostic sensitivity was 74%; for those that touched the visceral pleura, however, it was only 35%. They compared these results to 121 cases of CT-guided needle biopsy where the lesions averaged 37 mm in greatest dimension. The overall diagnostic sensitivity was 64%. There were greater complications with 27 of the 121 (22.3%) patients developing a pneumothorax following the transthoracic biopsy, and of these 121 patients 8 (7%) required intercostal catheter placement. In contrast to the 140 patients who underwent bronchoscopic biopsy, only 2 (1.5%) developed a pneumothorax and none required catheter placement. Connor and colleagues29 reviewed 106 transthoracic needle biopsies performed on 103 patients for their diagnostic accuracy and complication rates. If a diagnosis could not be established on the transthoracic needle biopsy, the patient underwent another procedure until the diagnosis was established. Eighty-five patients had malignant lesions, of which 75 (88%) were diagnosed on biopsy. Eighteen patients had benign lesions, of which 12 (67%) were diagnosed on biopsy. As for complications, Connor and colleagues29 reported a 19% overall pneumothorax rate, but only 2.4% of those pneumothoraces were large (>30%), requiring chest drainage. As for hemorrhage, only 3.8% of the patients reported hemoptysis.
Open-Lung or Video-Assisted Thoracic Surgery (VATS) Biopsy
The open-lung biopsy or the video-assisted thoracic surgery (VATS) biopsy provides much more pulmonary tissue for examination than either the transbronchial or the needle biopsy. With an open-lung biopsy or a VATS procedure, the surgeon can explore at least part of the chest cavity, palpate the lung, and select an appropriate area for biopsy. Bensard,12 Kadokura,66 and Allen5 and their colleagues reported that the diagnostic yield from a VATS procedure is equivalent to that from an open-lung biopsy. The specimens produced from this type of procedure vary in size from 20 to 60 mm in greatest dimension. Depending on the clinical diagnosis, the specimen may be handled according to Figure 15-1. The biopsy tissue is divided into two separate specimens, one for microbiology and one for anatomic pathology. The microbiology specimen can be taken by either the pathologist or the thoracic surgeon. The surgeon is
operating under sterile conditions and is in a better position to obtain an uncontaminated specimen than is the pathologist.
operating under sterile conditions and is in a better position to obtain an uncontaminated specimen than is the pathologist.
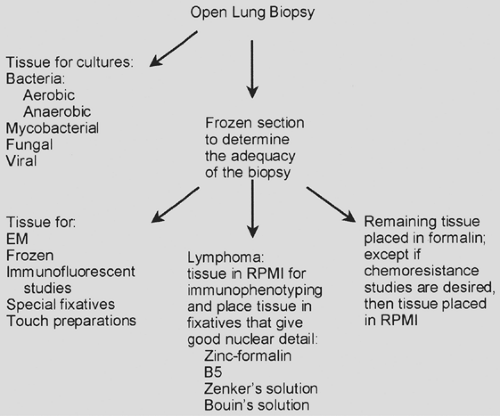 Figure 15-1. Handling of an open-lung or video-assisted thoracoscopic biopsy. EM, electron microscopy. RPMI Roswell Park Memorial Institute, tissue culture media. |
The appropriate biopsy site depends on the location of the lesion. Gaensler and Carrington45 recommended avoiding taking a biopsy from the lingua. Since then, the tips of the lung have been avoided. These areas may show fibrosis and they are difficult to interpret in the context of diffuse interstitial or vascular lung disease. The investigators believe that it normally shows scarring and chronic inflammation, which makes it difficult to diagnose interstitial lung disease. Temes and colleagues133 addressed the issue of performing a biopsy on the lingula for interstitial lung disease. They found the lingula to have a diagnostic yield equivalent to that from other sites.
Frozen Section
The specimen for anatomic pathology should be examined by the pathologist before the end of the operation. A frozen section should be avoided if at all possible, but it ensures the surgeon of an adequate specimen and provides a preliminary diagnosis. The tissue should be approached with a fresh blade. A sharp blade will minimize the crush artifact. Based on the results of a frozen section, the pathologist can determine what other steps can be taken to arrive at the proper diagnosis. For instance, tissue can be placed in glutaraldehyde for electron microscopy, and this tissue should measure less than 1 mm. Electron microscopy has, in most instances, been replaced by immunohistochemistry. Electron microscopy, however, may be useful in detecting the electron-dense material of immune complexes or amyloid. Amyloid may be detected in tissue that has not been fixed in glutaraldehyde. Tissue may also be placed in Michelle’s solution for immunofluorescent studies. These studies would be useful for such diseases as Goodpasture’s syndrome. Tissue may be frozen for special studies, such as immunohistochemistry, gene rearrangement studies, or some other study, but most of these studies can now be performed on formalin-fixed paraffin-embedded tissue. Air-dried and fixed (95% alcohol) touch preparations can be prepared. Fresh tissue may be sent for cytogenetic studies.
If the frozen section suggests a lymphoma, tissue can be placed in Roswell Park Memorial Institute (RPMI) medium (a tissue culture medium), and immunophenotyping studies can be performed by flow cytometry. If the lesion is a lymphoma or a plasma cell tumor, the immunophenotyping should yield a monoclonal population of cells. In addition, tissue may be placed in “hematopathology” fixatives that yield good nuclear detail, such as zinc-formalin, B5, Zenker’s solution, or Bouin’s solution. The remaining tissue should be placed in routine (10%) formalin solution. According to Churg,24 a small syringe with a small-gauge needle can be used to inject formalin into the specimen. The injection of formalin will both preserve the architecture and fix the lung tissue. If the specimen has been cut, it may help to reduce the crush artifact. If the specimen requires a frozen section, diluted frozen section compound may be injected into the tissue. This may decrease the crush artifact created by cutting. In many instances, the surgeon will place the lung tissue in formalin, but the formalin will not penetrate into the center of the tissue.
Segmentectomy, Lobectomy, and Pneumonectomy
After the open-lung biopsy, the larger pulmonary specimens include segmentectomies, lobectomies, and pneumonectomies. These larger specimens are usually the result of a therapeutic maneuver rather than a diagnostic one. Consequently they are
handled differently from an open-lung biopsy. If the lung resection was for a tumor, the pathologist may be called to perform a frozen section on the bronchial margin. The frozen section ensures the surgeon that his margin is free of tumor. If indicated, the pathologist may also place tissue in RPMI medium for drug-resistance studies or in special fixatives (Fig. 15-1).
handled differently from an open-lung biopsy. If the lung resection was for a tumor, the pathologist may be called to perform a frozen section on the bronchial margin. The frozen section ensures the surgeon that his margin is free of tumor. If indicated, the pathologist may also place tissue in RPMI medium for drug-resistance studies or in special fixatives (Fig. 15-1).
Corrin31 describes how the lung may be inflated with formalin. This is accomplished by placing a rubber tube into the main bronchus and securing the tube in place. The tube is connected to a container of formalin. The formalin is allowed to flow into the lung or lobe at a height of about 25 cm. After the lung is inflated, the tube is removed and the bronchus is clamped with a hemostat. The specimen is placed in a deep pan filled with formalin. If the lung floats, it is covered with gauze or paper towels, which touch the formalin and serve as a wick. The surface of the specimen remains moist.
After about 4 hours, the specimen should be sufficiently fixed to be cut. Cutting or opening the specimen may be done in a variety of ways. The most common is by opening the bronchi with a pair of scissors. Corrin31 describes a method popularized by the late A.A. Leibow from Yale. A long probe is inserted down the bronchus until it perforates the visceral pleura. With the probe serving as a guide, a knife slices off the lung tissue above the probe. The cut lung tissue is removed, and this exposes the bronchial tree. This method can also be used to provide a lobectomy specimen. The segmentectomy specimens are small, and it may be easier to dissect out the bronchi with a pair of scissors.
Cytology Specimens
Pulmonary cytology specimens vary considerably in type (Table 15-1). The specimens range from the exfoliated cells of sputum to the retrieved specimens of lavages, brushings, and CT-guided fine-needle aspirations. Pleural fluid is a form of retrieved specimen. Cytology specimens are usually processed for a cell block and a cytospin preparation. A cytospin preparation consists of placing a small amount of well-mixed fluid (pleural, sputum) in a cytocentrifuge and centrifuging the cells onto a glass slide. The cells may be stained with Papanicolaou’s, Wright–Giemsa, and H&E stains or a combination of these stains. The cell block is processed in a similar manner to tissue, and a histologic section is prepared from the block. The cell block and the cytospin are examined for malignant cells or whatever else the clinician suspects. Special stains and immunohistochemistry can be performed on the cell block.
Pleural Fluid
The pleural cavity normally contains a small amount of fluid that serves as a lubricant between the chest wall and the lung. Smith and Kjeldsberg118 divide pleural fluids into transudates and exudates. Transudates are due to increased hydrostatic pressure and usually occur in the setting of congestive heart failure. Exudates are due to increased capillary permeability or decreased resorption. They may be due to infections, neoplasm, pulmonary infarcts, or other pleural diseases. Pleural fluid must be sent for appropriate studies, which can include cultures, chemistry, cell count, and cytologic examination. Cytologic examination includes a cytospin preparation and a cell block. Heffner and Klein57 reviewed malignant pleural effusions. The most common types of malignant effusions are lymphomas, mesotheliomas, and carcinomas of the breast, lung, gastrointestinal tract, and ovaries.
Sputum
Sputum is a mixture of mucus, exfoliated cells, and alveolar macrophages, but it may also contain inflammatory cells, squamous cells (oropharyngeal contamination), and debris. An adequate specimen must contain alveolar macrophages. Sputum is used to diagnose pneumonia or a malignancy. For pneumonia, the sputum is sent to microbiology for culture and Gram’s stain. For a malignancy, the sputum is sent to cytology, where the specimen is cytocentrifuged and a cell block made. Cytologic examination of the sputum may be helpful. Because sputum is a passively acquired specimen, it is used for diagnosing malignancies. However, with the advent of bronchoscopy and fine-needle biopsy and aspiration, it is used less and less to diagnose malignancies. Sputum cytology was found to be ineffective in a National Cancer Institute trial for early lung cancer detection. In contrast, Petty91 describes a community lung cancer detection program in which sputum cytology examinations were useful in detecting lung cancer in its early stages in high-risk patients.
Bronchoalveolar Lavage
Meyer85 reviewed the use of BAL as a diagnostic tool. It can aid in the diagnosis and management of a variety of lung disorders including malignancy, interstitial disease, and pulmonary infections. If they are involved with the disease process, the right middle lobe and the lingula represent easily accessible parts of the lung. The distal end of the bronchoscope can be wedged into a segmental or subsegmental bronchus, and this action avoids contamination of the BAL with proximal airway secretions. Protocols for BAL use six aliquots that range from 20 to 60 mL of normal saline; they are generally warmed to 37°C just prior to instillation. The first aliquot is considered to represent airway cells and secretions and is sent for microbiology. The subsequent aliquots are considered to represent the distal airspaces and are pooled for cell analysis. Between 300 and 500 white blood cells should be counted. The average differential count for a nonsmoker should contain mostly alveolar macrophages (80%–90%), some lymphocytes (5%–15%), and very few neutrophils (<3%) or eosinophils (<1%). According to Meyer85 the differential of the BAL cell count is useful in categorizing the type of pulmonary disease. A lymphocyte count of >25% suggests a granulomatous lung disease, drug reaction, cellular nonspecific interstitial pneumonitis (NSIP), or viral infection. A lymphocyte count of >50% suggests hypersensitivity pneumonitis, drug reaction, or NSIP. An eosinophil count of >25% indicates eosinophilic lung disease. A neutrophil count of >50% supports a diagnosis of acute lung injury or suppurative lung infection. BAL fluid can be useful in identifying infectious organisms, particularly in the resuspended cell pellet. The suspension can be used for cultures as well as staining for microorganisms (Table 15-3).
The fluid from a BAL should be processed in a standardized way. A cell count should be performed with a hemocytometer. Cytology should be performed as described for the cytology
specimens. In addition to the stains mentioned, the Diff-Quik (Baxter Diagnostics, Inc., McGaw Park, IL) may also be used. The BAL fluid may be used for a variety of chemical analyses. The specimens should be processed as rapidly as possible, which is usually within 4 hours. Otherwise, the samples should be stored at 4°C, and they may be kept for up to 24 hours. With delayed processing, the cell counts may be decreased but the differential is usually the same.
specimens. In addition to the stains mentioned, the Diff-Quik (Baxter Diagnostics, Inc., McGaw Park, IL) may also be used. The BAL fluid may be used for a variety of chemical analyses. The specimens should be processed as rapidly as possible, which is usually within 4 hours. Otherwise, the samples should be stored at 4°C, and they may be kept for up to 24 hours. With delayed processing, the cell counts may be decreased but the differential is usually the same.
Table 15-3 Pulmonary Conditions in Which Bronchoalveolar Lavage May Be Useful | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Reynolds97 pointed out that by the late 1980s, BAL fluid was being used to recover microbial organisms, especially Pneumocystis jirovecii (formerly known as Pneumocystis carinii) in AIDS patients as well as to study other diseases, such as asthma, occupational lung diseases, acute lung injury, and the complications of organ transplantation. Goldstein and associates50 of the American Thoracic Society pointed out that BAL is useful for making a diagnosis of carcinoma or metastatic carcinoma as well as other diseases, such as alveolar proteinosis, eosinophilic granuloma, eosinophilic pneumonia, and certain pneumoconiosis.
BAL fluid cell counts may be helpful in diagnosing interstitial lung disease, but Welker and colleagues144 emphasize that it is not diagnostic. They reviewed 3,975 BAL specimens from 3,118 patients and categorized the specimens based on the clinical picture as to whether they represented interstitial lung disease, inflammatory disease, lung tumor mimicking interstitial lung disease, exposure to dust and fibers, and others. The first three groups with a total of 1,748 patients were chosen for analysis. In 583 patients (33.4%), the final diagnosis was inflammation; 455 patients (26%) had a benign or malignant tumor; and 685 patients (34.6%) had interstitial lung disease. The most common interstitial lung diseases were sarcoidosis, usual interstitial pneumonia (UIP), NSIP, and extrinsic allergic alveolitis (EAA). These four diseases made up about 60% of all interstitial lung diseases. They concluded that BAL cell counts were not specific for a disease except in the detection of infection or tumor cells and that they were useful only in the most common interstitial lung diseases. They did find that an elevated CD4/CD8 cell ratio was more indicative of sarcoidosis and that the cell counts need to be viewed in the context of other clinical findings. In another review of BAL, Kilinç and Kolsuk74 concluded that BAL was supportive of the diagnosis of the most common interstitial lung diseases, such as sarcoidosis, extrinsic allergic alveolitis, and UIP, but that in all instances the diagnosis needed to be correlated with the clinical and radiographic findings.
In contrast, BAL is very useful in managing lung transplant recipients. Tiroke and colleagues136 described the normal BAL findings in a transplanted lung as well as the abnormal findings in a reimplantation response, acute and chronic rejections, and infections. The reimplantation response occurs in the first week after transplantation, and the BAL contains a markedly increased number of neutrophils. The reimplantation response is a reversible organ dysfunction, probably brought about as a response to ischemia and reperfusion. Acute rejection usually occurs in the first week after transplantation and is characterized by an increased cell count in the BAL and lymphocytosis. The histology of chronic rejection is obliterative bronchiolitis; this is characterized by submucosal fibrosis of the respiratory bronchioles that results in complete or near complete occlusion of the bronchiolar lumen. This diagnosis can be made only on tissue biopsy, which may be problematic in a lung transplant patient. In chronic rejection, the BAL shows an increased cell count with both lymphocytes and neutrophils. It may also show in such factors as IL-8, antioxidants, and others. In the proper clinical setting, this cytology is suspicious for a chronic rejection. Bowdish and colleagues16 discussed the bronchiolitis obliterans syndrome (BOS) as a surrogate marker obliterative bronchiolitis. This syndrome is characterized by varying degrees of reduction in the forced expiratory volume in 1 second (FEV1); at 5 to 7 years following transplantation, up to 80% of patients may develop BOS.
Bronchial Brushing
Metersky and Gupta84 described bronchial brushing as passing a sheathed brush down a flexible bronchoscope and then passing the brush over the lesion. Cells or organisms are trapped in the bristles of the brush. The sheathed brush is then pulled out through the bronchoscope. The cells on the brush are smeared out on glass slides. Some slides are immediately placed in 95% alcohol and others are allowed to air dry. Sato and colleagues108 found that brushes with bristles that measured less than 0.1 cm in diameter collected more cells and showed less air-drying artifact. Gaber and associates44 performed bronchial biopsy, conventional brushing, lavage, and whole endobronchial brushing on 39 patients with endoscopically visible lung tumors. The whole endobronchial brush was cut off and placed in Shandon’s cytospin collection fluid. The cells were mechanically separated from the brush and cytospin preparations were made from the fluid. The results were positive for malignant cells in 31 (79%) patients on bronchial biopsy, 29 (74%) on conventional brushing, 21 (54%) on lavage, and 16 (41%) on whole brush. This study shows that bronchial biopsy is generally superior to other sampling forms.
Lung Fine-Needle Aspiration
Fine-needle aspirations of the lung are performed in a manner similar to transthoracic needle biopsies. The technique for transthoracic needle biopsy was described earlier. Klein and Zarka73 described the technique for needle aspirations. The aspiration needle can be inserted into the lesion either through a guide needle or by itself. After the imaging studies confirm the presence of the needle in the lesion, a 10- or 20-mL syringe is attached to the needle or to a plastic tube that is attached to the needle and then aspirated. The needle is usually moved in
an up-and-down or rotary motion, piercing the lesion multiple times, during which suction is applied. After 2 to 5 seconds, the needle is removed and the aspirated tissue placed in normal saline or smeared out on microscopic slides. If a guide needle is present, aspiration can be performed more than once. The fluid from the tip of the needle is smeared on the slides. The slides are both air-dried and immediately fixed in 95% alcohol. The needle, tubing, and syringe are rinsed with physiologic saline. The fixed smears are stained with H&E and Papanicolaou’s stains, whereas the air-dried smears are stained with Wright–Giemsa stain. The material in physiologic saline is cytocentrifuged to produce cytospin smears. If sufficient tissue is present, the remaining fluid can be used to make a cell block. Transbronchial needle aspirations can also be performed, with the material processed in a similar manner. They have the same complications of pneumothorax and hemorrhage as needle biopsies of the lung.
an up-and-down or rotary motion, piercing the lesion multiple times, during which suction is applied. After 2 to 5 seconds, the needle is removed and the aspirated tissue placed in normal saline or smeared out on microscopic slides. If a guide needle is present, aspiration can be performed more than once. The fluid from the tip of the needle is smeared on the slides. The slides are both air-dried and immediately fixed in 95% alcohol. The needle, tubing, and syringe are rinsed with physiologic saline. The fixed smears are stained with H&E and Papanicolaou’s stains, whereas the air-dried smears are stained with Wright–Giemsa stain. The material in physiologic saline is cytocentrifuged to produce cytospin smears. If sufficient tissue is present, the remaining fluid can be used to make a cell block. Transbronchial needle aspirations can also be performed, with the material processed in a similar manner. They have the same complications of pneumothorax and hemorrhage as needle biopsies of the lung.
The value of transthoracic fine-needle aspirations as opposed to transthoracic needle biopsies is uncertain. Greif and colleagues52 compared the result of transthoracic needle biopsies with fine-needle aspiration from 156 patients. Both biopsies were performed sequentially at the same visit. The aspiration provided a diagnosis in 133 (85.3%) of the cases, and the needle biopsy provided a diagnosis in 121 (77.6%). The needle biopsy confirmed the aspirate in 90 (57.7%) patients, provided additional information in 17 (10.9%) patients, and was less informative than aspiration in 35 (22.4%) patients. The investigators concluded that needle biopsies offered no advantage over aspiration for peripheral lung lesions. In contrast, Yilmaz and associates156 performed fine-needle aspirations in 129 patients with lung nodules who subsequently underwent thoracotomy. They found a concordance of 73.6% between the aspiration diagnoses and the thoracotomy diagnoses. The poorest agreement was for large-cell carcinomas. If the tumors were well differentiated, there was a high degree of agreement between the aspirate and the tissue. It was concluded that aspirations did not always lead to the correct tumor types. In one final study, Delgado and coworkers36 found that aspirations were highly sensitive in distinguishing small-cell carcinoma from non-small-cell carcinoma. They concluded that aspiration is useful in selecting the appropriate modality to treat lung neoplasms.
Cytology
Value of Pulmonary Cytology
Pulmonary cytology has become a valuable adjunct in the diagnosis of pulmonary diseases. In the evaluation of solitary pulmonary nodules that measure less than 3 cm in greatest dimension, Winer-Muram149 described fine-needle aspiration as being useful. The resulting diagnoses generally fell into one of three categories: malignant, specific benign, or nonspecific benign. The categories of malignant and specific benign have the treatment dictated by the diagnosis. Those that show a nonspecific benign condition need further evaluation. In this instance, a core needle biopsy is more helpful in establishing a specific benign diagnosis (69% of patients) as opposed to a fine-needle aspiration (31% of patients). Patients who have a nonspecific benign diagnosis need both clinical and radiographic follow-up. If the lesion should grow over time, a repeat biopsy or a resection is indicated.
Crapanzano and Zakowski32 believe that the cytologist must interpret the cytologic specimen in the context of the clinical history and the radiographic findings. They further caution that rendering a diagnosis of malignancy in the presence of reactive pulmonary processes, infections, or infarcts may be difficult. The difficulty lies in squamous metaplasia looking like squamous carcinoma, and reactive type 2 pneumocytes being mistaken for adenocarcinoma. Nodit and colleagues92 considered the cytology errors as falling into either interpretive errors or sampling errors. The interpretive error would occur if the cytopathologist made a false-positive or false-negative diagnosis. The sampling error would occur if the malignant cells were not present in the sample.
Policarpio-Nicolas and Wick98 suggested that the overall sensitivity and specificity of pulmonary cytology ranged from about 60% to 96%. Much like Crapanzano and Zakowski,32 they focused on the causes for false-positive results in pulmonary cytology, describing the mimics of adenocarcinoma, squamous cell carcinoma, and small-cell carcinoma (Table 15-4). Ganjei-Azar and Nadji46 published a book about the role of immunohistochemistry in cytologic interpretation in which they included a pulmonary section. Immunohistochemistry should decrease the interpretative errors.
Pulmonary Cytology
Amy8 reviewed the cytopathology of pulmonary lesions. Pulmonary cytology is used in the diagnosis of pulmonary neoplasms and infections. This brief discussion focuses on the cytology of the more common pulmonary neoplasms, including small-cell carcinoma, adenocarcinoma, large-cell carcinoma,
and squamous cell carcinoma. In sputum cytology, the sensitivity for the detection of lung carcinoma is 64.5%, with a specificity of 99.5% and a negative predictive value of 98.2%. The sensitivity of fine-needle aspiration in the diagnosis is greater than that of sputum, with the sensitivity varying from 84% to 90%, the specificity from 95% to 100%, and the negative predictive value from 77% to 53.3%.
and squamous cell carcinoma. In sputum cytology, the sensitivity for the detection of lung carcinoma is 64.5%, with a specificity of 99.5% and a negative predictive value of 98.2%. The sensitivity of fine-needle aspiration in the diagnosis is greater than that of sputum, with the sensitivity varying from 84% to 90%, the specificity from 95% to 100%, and the negative predictive value from 77% to 53.3%.
Table 15-4 Pathologic Conditions That May Mimic Cytologic Malignancy | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
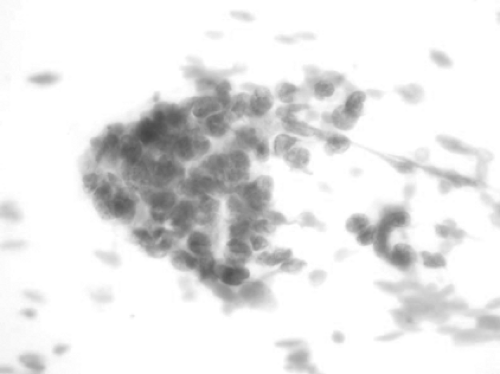
Pulmonary small-cell carcinoma is a high-grade malignant epithelial neoplasm. Cytologically, the neoplastic cells have scant cytoplasm and small nuclei that mold to each other. The nuclei have fine nuclear chromatin with inconspicuous nucleoli. The nuclei may show a crush artifact (Fig. 15-2). The cytologic differential diagnosis includes carcinoid, basal cell hyperplasia, lymphocytes, lymphoma, small-cell adenocarcinoma, basaloid squamous cell carcinoma, and various other entities. Most small-cell carcinomas are positive for thyroid transcription factor-1 (TTF-1) as well as synaptophysin, with about half showing positivity for chromogranin. The broad-spectrum cytokeratin cocktail may show a focal “dot-like” positivity in the cytoplasm.
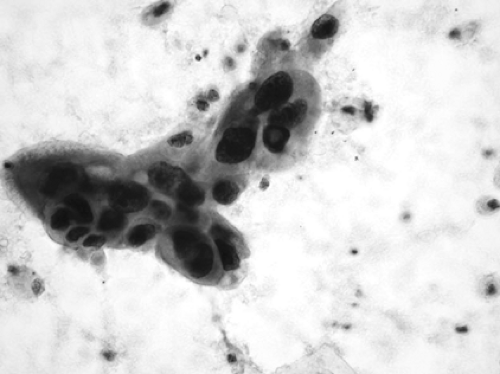
Adenocarcinoma is composed of clusters of epithelial cells with enlarged nuclei. The nuclei may have prominent nucleoli and the contour of the nucleus may be irregular. The cytoplasm is usually rounded and may or may not contain mucin (Fig. 15-3). Cytologically, adenocarcinomas may be impossible to differentiate from bronchioloalveolar carcinoma. Moran89 considers the diagnosis of bronchioloalveolar carcinoma to be limited to cases where there is a complete surgical removal of the neoplasm, and he does not consider it a cytologic diagnosis. The differential diagnosis includes reactive bronchiolar epithelium, well-differentiated adenocarcinoma, metastatic adenocarcinomas, and several other benign and malignant neoplasms. The nonmucinous adenocarcinomas will immunostain for TTF-1, whereas the mucinous ones will not.
Large-cell carcinomas are composed of cells with large nuclei and prominent nucleoli and a moderate amount of cytoplasm. The cells show no evidence of either squamous or glandular differentiation and in many cases are actually one of these two tumors with no evidence of differentiation. On histology, a large-cell carcinoma is defined as one that lacks features of glandular or squamous differentiation, with the definition based on light microscopy. In histology, there are two variants of the large-cell carcinoma: these are the large-cell neuroendocrine carcinoma and the basaloid carcinoma. Immunocytochemical stains may help in further subclassifying these tumors.
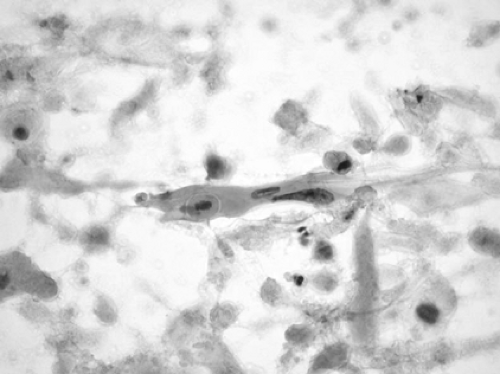
Squamous cell carcinoma is composed of cells with keratinized eosinophilic cytoplasm and hyperchromatic irregularly shaped nuclei. There is usually a background of necrotic and keratinous debris. Occasionally, intercellular bridges may be identified. The less mature cells may have more rounded, less hyperchromatic nuclei, more obvious nucleoli, and dense basophilic or amphophilic cytoplasm with well-defined borders (Fig. 15-4).
The immunocytochemical stain for TTF-1 is negative and the one for p63 is positive.
The immunocytochemical stain for TTF-1 is negative and the one for p63 is positive.
Special Stains
Special stains defy a neat classification. Anatomic pathology has a certain degree of subjectivity that requires judgment on the part of the pathologist. The purpose of special stains is to confirm a morphology judgment and to make that judgment less subjective. Special stains can be divided into histochemical and immunohistochemical. The histochemical stains can be for organisms (Tables 15-5 and 15-6) or for other purposes, such as a trichrome stain for collagen. Immunohistochemical staining techniques are more objective, sensitive, and specific, with an antibody binding to a specific protein, such as an antibody binding to insulin. These techniques are discussed in Chapter 168 as well as in the book by Taylor and Cotes.126 Table 15-7 lists useful immunohistochemical stains for pulmonary tumors.
Routine Special Stains
Routine special stains can be divided into those for microorganisms and those for other purposes, such as an iron stain. The Ziehl–Neelsen stain for mycobacteria and the Grocott–Gomori methenamine silver (GMS) stain for fungi are probably the most commonly used. The Ziehl–Neelsen stain makes mycobacteria red, and Mycobacterium tuberculosis has the appearance of a beaded rod. GMS stains the walls of fungi and Pneumocystis species black. Cryptococcus species stain black with GMS, but they also have a mucinous capsule that will stain pink with a mucicarmine stain. Despite the advances in immunohistochemistry, these routine special stains are very useful.
Immunohistochemical Stains
Brambilla and associates17 developed the World Health Organization classification of lung tumors based on the histology of the tumors as seen by light microscopy. They point out that immunohistochemistry is required for the diagnosis of large-cell neuroendocrine carcinoma and for differentiating between malignant mesothelioma and adenocarcinoma. In addition, immunostaining with HMB45 is helpful in clear-cell tumors and lymphangioleiomyomatosis, since they are positive. Finally, thyroid transcription factor–1 (TTF-1) is positive in many types of lung cancers. Marson and coworkers81 note that more than 90% of lung cancers are composed of four basic groups: adenocarcinoma (30%–40%), squamous cell carcinoma (30%), small-cell carcinomas (20%–25%), and large-cell carcinomas (10%–20%). In most instances, the type of lung carcinoma can be determined by routine light microscopy, but immunohistochemical stains may be useful in confirming the diagnosis or in cases where the histology is confusing. Even typical tumors do not always immunostain with the expected antibodies. The current discussion
focuses on the antibodies that are most useful in the immuno- staining of lung carcinomas. The antibodies for mesotheliomas are discussed in Chapter 68. Beasley11 reviewed the antibodies for lung carcinomas. The most useful ones are pankeratin cocktails with a mixture of several keratin antibodies to different-size keratins; thyroid transcription factor–1 (TTF-1); cytokeratin (CK) subsets 5/6, 7, and 20; p63; chromogranin; synaptophysin; and neural cell adhesion molecule (NCAM), which is also known as CD56 (Table 15-7).
focuses on the antibodies that are most useful in the immuno- staining of lung carcinomas. The antibodies for mesotheliomas are discussed in Chapter 68. Beasley11 reviewed the antibodies for lung carcinomas. The most useful ones are pankeratin cocktails with a mixture of several keratin antibodies to different-size keratins; thyroid transcription factor–1 (TTF-1); cytokeratin (CK) subsets 5/6, 7, and 20; p63; chromogranin; synaptophysin; and neural cell adhesion molecule (NCAM), which is also known as CD56 (Table 15-7).
Table 15-5 Histochemical Stains for Infectious Agents | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Table 15-6 Histochemical Stains for Noninfectious Processes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 15-7 Useful Pulmonary Immunohistochemical Stains | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Thyroid Transcription Factor–1
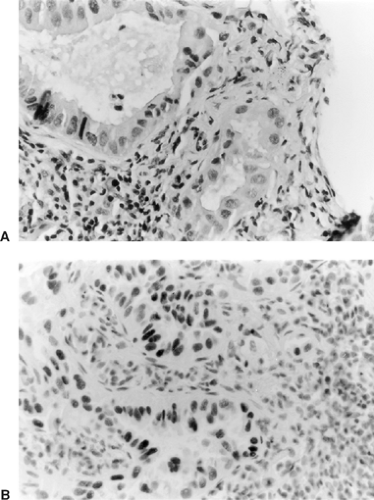
Marson and associates81 describe TTF-1 as a member of the NKX-2 gene family of the homeodomain containing nuclear transcription factors. A transcription factor is a protein that binds to specific sequence of DNA and controls the transcription of genetic information from DNA to RNA. TTF-1 is found in the thyroid, brain, and lung. In lung cancers, it is positive in nonmucinous adenocarcinomas (Fig. 15-5), nonmucinous bronchioloalveolar carcinomas, small-cell carcinoma, and large-cell neuroendocrine carcinomas. Surprisingly, it does not stain carcinoids or atypical carcinoids. If there is a consideration of metastatic thyroid carcinoma, an immunostain for thyroglobulin must be performed.
Cytokeratin
Cytokeratins (CKs) are the major intermediate filaments of epithelial cells and comprise a family of about 20 different polypeptides that range in size from 40 to 67 kDa. There are pankeratin mixtures of several antikeratin antibodies as well as antibodies to specific CKs such as 5/6, 7, and 20. Certain CKs are confined to certain epithelial cells. Kargi and colleagues68 used CK 5/6 and found that it, along with p63, consistently stained pulmonary squamous cell carcinomas. TTF-1 did not stain these carcinomas. CKs 7 and 20 are usually used together. Su and associates127 used them in conjunction with TTF-1 to distinguish between primary and metastatic adenocarcinoma in the lung. CK7 is found in lung and breast epithelium, whereas CK20 is common to the intestinal tract. The investigators found that the majority of metastatic colonic adenocarcinomas were CK20-positive but CK7- and TTF-1–negative, whereas the primary pulmonary adenocarcinomas were TTF-1– and CK-7–positive but CK20-negative.
p63
The p63 gene codes for six proteins that are thought to act as dominant-negative blockers of p53 proteins. Normally found in the reserve cells of ciliated bronchial epithelium, p63 has been postulated to have a critical role in maintaining squamous epithelium.
Wu and colleagues154 used p63 and TTF-1 to differentiate pulmonary small-cell carcinoma from poorly differentiated squamous cell carcinoma. All their cases of poorly differentiated squamous cell carcinoma were p63-positive and TTF-1–negative. In contrast, the pulmonary small-cell carcinomas were mostly positive for TTF-1 but negative for p63.
Chromogranin
The chromogranins are a family of acidic glycoproteins normally found in the matrix of dense-core granules in the neuroendocrine cells. Consequently the more granules present, the stronger the immunostain. Chromogranin would be expected to stain the four pulmonary neuroendocrine tumors, which include carcinoids, atypical carcinoids, large neuroendocrine tumors, and small-cell carcinomas. The degree of immunostaining would depend on the density of the neurosecretory granules.
Synaptophysin
Hammond and colleagues54 describe synaptophysin as a transmembrane protein found in the presynaptic vesicles of neural cells. This protein is important for calcium-mediated neurotransmission. The protein is variably expressed by the four pulmonary neuroendocrine tumors.
Neural Cell Adhesion Molecule (NCAM) or CD56
According to Lantuejoul and colleagues,77 neural cell adhesion molecules belong to the immunoglobulin family of cell-surface adhesion proteins involved in direct cell–cell adhesion and are expressed in neuroendocrine tissues and tumors. They immunostained 120 neuroendocrine tumors, including carcinoid, atypical carcinoid, small-cell carcinoma, and large-cell
neuroendocrine carcinoma, with all being positive for NCAM. In addition, they immunostained 25 squamous cell carcinomas and 25 adenocarcinomas for NCAM, and all were negative.
neuroendocrine carcinoma, with all being positive for NCAM. In addition, they immunostained 25 squamous cell carcinomas and 25 adenocarcinomas for NCAM, and all were negative.
Molecular Pathology
This section introduces the reader to some basic concepts of molecular pathology and how they apply to the laboratory; it also discusses the terminology of molecular biology. Molecular pathology includes both anatomic and clinical pathology. The latter part of the chapter focuses on specific pulmonary infectious agents and discusses some of the molecular methods used to diagnose them. The molecular biology of lung carcinoma is covered in Chapter 106. In anatomic pathology, most of the clinical testing for the molecular markers of carcinomas is done by immunostaining, which is described in Chapter 168.
Terminology of Molecular Biology
Human cells contain 23 pairs of chromosomes. Of these, 22 are autosomes and one pair comprises the sex chromosomes, XX in the female and XY in the male. The chromosomes, located in the cell nucleus, are composed of DNA and encode the genetic information of the organism. Each chromosome bears a linear array of genes. The 22 autosomal chromosomes are paired, and each autosomal locus is represented twice. An allele is any particular form of a gene that can exist at a particular locus. If both chromosomes have the identical gene or allele at the same locus, the genes are referred to as homozygous alleles. If the genes are not identical at the same locus, they are referred to as heterozygous alleles.
DNA exists in the form of a double helix: when it is transcribed into messenger RNA (mRNA), it unwinds. The transcription requires three RNA polymerases as well as proteins known as transcription factors. Santis and Evans,106,107 in their review of molecular biology, point out that DNA and mRNA are not collinear. In other words, the mRNA is not complementary to the DNA because the DNA contains exons (expressed sequences) and introns (noncoding sequences). The introns are removed from the final sequence of the mRNA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree