Chapter 28 Kidney and Pancreas Transplantation
Historical Perspective
Interest in transplanting organs into humans dates back to the early 1900s. Floresco described anastomosis of the renal graft to the iliac fossa in 1905. In 1906, Jaboulay attempted to use a pig kidney to cure a patient with acute nephritis. He anastomosed the renal xenograft to the brachial arteries of the patient and urine was noted for 1 hour postreperfusion. Alexis Carrel was developing techniques of triangulation of vascular anastomoses by performing various organ transplants in animals and received the Nobel Prize in 1912. However, organ function was minimal and further attempts at organ transplantation were abandoned. However, in the early 1950s, Medawar and colleagues described the prevention of rejection in mice and human organ transplantation was again attempted. The first successful renal transplantation was performed by Murray in 1954 between identical twins. Other major milestones in transplantation have included the discovery of cyclosporine and other effective immunosuppressive medications, description of the histocompatibility antigens, and perfecting of preservation solutions (Table 28-1).
Table 28-1 Major Milestones in the History of Transplantation
| YEAR | MILESTONE |
|---|---|
| 1954 | Dr. Joseph Murray performs the first successful kidney transplantation between identical twins. |
| 1966 | Kelly and Lillihei perform the first pancreas transplantation. |
| 1967 | First simultaneous kidney and pancreas transplantation. |
| 1970s | Borel, Stahelin, Calne, and White initiate trials of use of cyclosporine in transplantation. |
| 1980s | Belzer and Southard develop University of Wisconsin Solution (Viaspan). |
| 1990 | Dr. Murray receives the Nobel Prize in Medicine. |
| 1990 | Scharp and Lacy report the first successful human clinical islet transplantation. |
Kidney Transplantation
Indications
Kidney transplantation offers patients better long-term outcomes than dialysis. The quality of life is improved and survival is projected to be 10 years longer than if the patient remains on dialysis.1 During the past decade, the kidney waiting list has grown and the death of candidates who die while waiting has doubled. This reflects a change in demographics in the recipient waiting list, with patients listed at older ages and an increasing number of patients being inactive on the waiting list.2
The most common causes of renal disease have evolved over the last 10 years. Overall, the percentage of patients with diabetes and hypertension as the cause of failure has increased from 24% to 28% and the percentage of glomerular disease has declined from 42% to 21%.2 In addition, the incidence of chronic kidney disease has also rapidly increased, from 209,000 patients in 1991 to 472,000 in 2004. Coresh and associates3 have noted that the higher prevalence of diabetes, hypertension, and higher body mass index (BMI) explains this trend. Similarly, the waiting list for kidney transplantation continues to grow every year. Potential recipients are also older than in past decades, with the group aged 50 to 64 years seeing the greatest increase (Fig. 28-1) This change in demographics has certainly presented challenges in preparation of these patients for transplantation and immunosuppression. It is also estimated that by 2015, the annual incidence of end-stage renal disease will be 136,000 patients/year and the prevalence will be 712,000 patients/year.
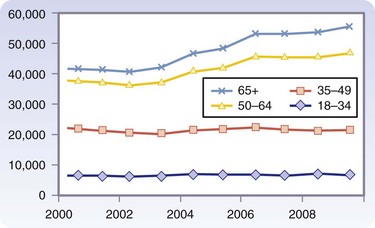
FIGURE 28-1 Additions to the UNOS-OPTN (Organ Procurement and Transplantation Network) waiting list by age.
(OPTN/UNOS Ethics Committee Report: Waiting list patient characteristics at end of year; kidney waiting list; active waitlist patients, 1999 to 2008 [http://www.ustransplant.org/annual_reports/current/501a_age_ki.htm]).
Patient Selection
The evaluation of patients as appropriate candidates for transplantation can be an arduous process. Patients with end-stage kidney disease have significant comorbidities and these must be taken into account when evaluating for transplantation. Guidelines for evaluation of these patients have been established.4 Emphasis should be placed on determining the original cause of renal disease so that the patient can be given reasonable expectations for graft survival. A graded association has been reported between reduced glomerular filtration rate (GFR) and risk of death and cardiovascular events.5 Mortality rates are more than 20%/year with dialysis. Long-term follow-up of kidney transplant recipients has shown a clear survival advantage over remaining on dialysis.6 Studies have also shown significant improvements in quality of life measures.7
The first step in the evaluation process is referral to a transplantation center. Many factors may affect the ability of the patient to be seen for evaluation. Furth and coworkers8 have shown that lower socioeconomic status, female gender, and lower level of education results in fewer referrals. There has been concern that geographic distance to a transplantation center might negatively influence access to care. However, a study of rural populations has shown that remote or rural residence is not associated with a longer waiting list time.
Recipients must be carefully evaluated for surgical risk as well as their ability to tolerate long-term immunosuppression. With improvements in perioperative management, the indications for kidney transplantation have increased. The absolute and relative contraindications for transplantation are shown in Box 28-1. HIV infection was once a contraindication to transplantation, but select patients have good results with transplantation as a treatment modality for HIV-associated nephropathy.9 According to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines, patients with a GFR of 30 mL/min/1.72 m2 or less, and/or chronic kidney disease (CKD), stage 3 or 4, should be referred to a nephrologist (http://www.kidney.org/professionals/kdoqi/index.cfm). Patients whose GFR falls below 20 should be evaluated as possible kidney transplant recipients if they do not have an absolute contraindication.
Common causes of renal failure leading to the need for replacement therapy include diabetes, hypertension, glomerular disease, interstitial disease, cystic disease, and chronic allograft nephropathy, with subsequent failure of a transplanted kidney. Kidney disease can recur in the allograft with varying frequency. Some diseases may lead to transplant failure with an inability to retransplant, such as aggressive focal sclerosing glomerulonephritis. Common primary renal diseases and their probability of recurrence are listed in Table 28-2.10–15
Table 28-2 Primary Renal Diseases and Recurrence Rates
| DISEASE | RECURRENCE RATE (%) | GRAFT LOSS (%) |
|---|---|---|
| Diabetes | 100 | Low until 10 yrs post-transplant |
| Focal segmental glomerulosclerosis | 20–30, first transplant; 80, second transplant | 40–50 |
| Membranoproliferative glomerulonephritis (MPGN) type 1 | 20–30 | 20–60 |
| MPGN type 2 | 50–100 | 10 |
| IgA nephropathy | 40–50 | 30 |
| Membranous nephropathy | 40 | Up to 50 |
| Hemolytic uremic syndrome | 30 | 20–30 |
| Systemic lupus | 30 | Rare |
| Polycystic kidney disease | 0 | 0 |
Screening of potential recipients should begin with a detailed history, paying particular attention to the original cause of disease. The length of time on dialysis has been noted to be an independent risk factor for poorer outcomes.16 The past medical history should include exposures to infectious diseases (especially tuberculosis, cytomegalovirus, Epstein-Barr virus, and hepatitis) as well as malignancy. Cardiac risk factors should be evaluated. A family history of renal disease or other systemic illnesses should be documented. Routine screening examinations such as Pap smear, mammography, colonoscopy, dental prophylaxis, and bone density scanning should be carried out as recommended by clinical practice guidelines. Prostate-specific antigen levels should be checked in men older than 50 years. In addition, the patient should be questioned about thrombotic events such as miscarriages, multiple dialysis access events, deep venous thrombosis, or pulmonary embolus so that a hypercoagulable profile can be obtained. The ability of the patient to tolerate immunosuppression should be evaluated. This not only involves consideration of the medical conditions, but also the ability to comply with a complex medical regimen and the financial ability to obtain the medications.
As noted, end-stage kidney disease patients are at increased risk for cardiovascular disease.5 Hence, a careful preoperative cardiac screening must be completed. However, there is little consensus about the optimal screening algorithm. Patients should have a baseline electrocardiogam (ECG) obtained, recognizing that almost 75% will have evidence of left ventricular hypertrophy. The patient’s risk profile should be assessed to see whether any risk factors can be modified (e.g., diet, weight management). Low-risk patients include those who have good functional capacity and no previously identified cardiac disease. These are typically patients with isolated renal disease such as immunoglobulin A (IgA) nephropathy or polycystic kidney disease, and with little comorbidity. Moderate-risk patients should undergo stress testing. It is important to ensure that the stress is diagnostic and a reasonable heart rate is achieved. Moderate-risk patients include those without cardiac symptoms but who have diabetes, prior history of heart disease, or two or more other risk factors for coronary disease (e.g., smoking, strong family history, hyperlipidemia, hypercholesterolemia). High-risk patients include those with a positive noninvasive test result, long-standing diabetes, or a history of severe congestive heart failure. These patients require cardiac catheterization prior to being accepted for the transplant list. Cardiac revascularization should occur prior to transplantation. If the patient requires lifelong clopidogrel (Plavix), there will be an increased risk of bleeding.17 Patients are required by federal guidelines to be reevaluated on a yearly basis. At any reevaluation, the patient’s cardiac status should be routinely reviewed and updated.
A full physical examination should be completed. Renal patients are at increased risk for cerebrovascular events18; therefore, if carotid bruits are discovered, patients should be screened for significant carotid stenosis. Atrial fibrillation can also be discovered on physical examination. The femoral, dorsalis pedis, and posterior tibial arteries should be palpated and any bruits documented. If the pulses are abnormal, or the patient has undergone previous amputation for vascular disease, noncontrast abdominal and pelvic CT scans should be obtained to assess the level of peripheral vascular disease. Iliac inflow might be significantly compromised, which would prevent the patient from having a successful outcome. If inflow is compromised, then one can consider whether a revascularization is warranted prior to or at the time of transplantation.19
Kidney organs can be obtained from living or deceased donors. The demand for kidney transplant and appropriate organs has continually increased given the increase in the burden of ESRD. Although living related donor (LRD) and living unrelated donor (LURD) numbers have increased in recent years, expanding the deceased donor pool is crucial. In 2003, the National Organ Breakthrough Collaborative was launched. The intent of this national effort was to increase the conversion rate (number of families consenting to donation in appropriate potential donors) to 75%. An update in 2005 sought to increase donors organs further by increasing the average organs transplanted/donor to 3.75.20 Deceased donor kidneys are placed in three broad categories: extended-criteria donor (ECD), standard criteria donor (SCD), and donor after cardiac death (DCD). As part of the effort to increase the pool of potential kidney organs, an emphasis was placed on ECD and DCD kidneys. In the past, these donor organs had a high rate of discard and there were no uniform DCD policies across the country. ECD kidneys are obtained from donors older than 60 years or from donors aged 50 to 59 years with at least two of the following criteria: cerebrovascular accident as cause of death, terminal creatinine higher than 1.5 mg/dL, or a history of hypertension. Kidneys from donors meeting the criteria for ECD have a 1.7 relative risk of graft loss when compared with kidneys from other donors.21 However, recipients of ECD kidneys clearly have a survival benefit when compared with those remaining on the waiting list.22
Living Donor Selection
The first successful living kidney donation was performed in 1954. Since that time, data continue to show that living kidney donation provides the best graft and patient survival results.23 Donors may or may not be genetically related to their intended recipient. In some cases, living donors are anonymous. There are now reports of extended altruistic donor chains. In these cases, an initial donor-recipient pair cannot go forward with transplantation usually because of ABO incompatibility or sensitization of the recipient. A reciprocal exchange with another incompatible pair allows for a domino transplantation, with multiple exchanges with as many as 10 kidney transplant chains reported.24 The 5-year survival of an unrelated kidney transplant is the same as that from a related donor. Interestingly, the outcome from a completely mismatched donor and one who is haploidentical is also similar. The underlying premise of living donation is that the donor will not suffer any medical consequences from the donation and has minimal surgical risk.
Currently accepted eligibility criteria include the following: age, 18 to 70 years, BMI less than 35, no cancer or active infection, and adequate renal function. ABO compatibility is also a consideration. However, recipients can undergo desensitization protocols and transplantation can be performed across ABO barriers. The donor should be informed in these circumstances of an increased risk of rejection of the kidney by the recipient. There is some individual variation among transplantation centers concerning acceptable GFR or BMI values. Relative contraindications include renal stones, impaired glucose tolerance, with a family history of type 2 diabetes, GFR of 70 to 80, hypertension, and BMI higher than 35. Absolute contraindications are listed in Box 28-2. For screening, all donors should have a thorough history and physical examination completed. Potential donors should be asked about nonsteroidal anti-inflammatory drug (NSAID) use in addition to questions about any medical illnesses. Potential donors should be made aware of the need to be away from work for a period of time and their willingness to donate free of coercion should be ascertained. An ECG and chest x-ray should be obtained. Routine laboratory work should include urinalysis, complete blood count (CBC), liver function tests, determination of creatinine (Cr) level, with estimated GFR (eGFR), lipid profile, microalbumin level, and oral glucose toleranc test. Prostate-specific antigen (PSA) levels should be obtained in men. Mammograms and Pap smears should be obtained in women of appropriate age. Radiographic evaluation of the anatomy of the renal arteries, veins, and collecting system should be performed and can be done via computed tomography (CT) angiography, magnetic resonance imaging (MRI), or arteriography, based on local expertise. In addition, all donors must be evaluated by an independent donor advocate (IDA). The IDA is not influenced by a relationship with the intended recipient or the transplantation center. The donor and recipient pair must also adhere to the National Organ Transplant Act of 1984, which states that “It is unlawful for any person to knowingly acquire, receive, or otherwise transfer any human organ for valuable consideration for use in human transplantation.” Many transplantation centers ask potential donors to undergo a psychological or psychiatric evaluation.
Potential donors should be informed that the risk of perioperative mortality, regardless of surgical technique, is approximately 0.03%.23 Matas and colleagues25 have surveyed 234 United Network for Organ Sharing (UNOS)-listed kidney transplantation programs and found that reoperation is required in 0.4% of patients undergoing open nephrectomy, 1.0% in hand-assisted laparoscopic nephrectomy, and 0.9% in total laparoscopic nephrectomy.
Laparoscopic Surgical Technique
The right or left kidney can be procured laparoscopically. The left renal anatomy is generally preferred because the renal vein is longer. Many studies have shown that the right kidney can be procured safely.23 A left kidney dissection is described here because it is more commonly done. A 5-mm entry site is placed in the left lower quadrant and a Veress needle is used to insufflate the abdomen to a pressure of 10 to 15 mm Hg. A 12-mm port is placed at the umbilicus. Two additional 5-mm ports are placed, one at the left costal margin and the last in the midaxillary line to retract the kidney.
Open Surgical Technique
The patient is placed in the lateral decubitus position. A subcostal incision is made from the tip of the 12th rib anteriorly, extending approximately 10 to 12 cm. The latissimus dorsi and posterior serratus are divided. The external and internal oblique muscles are divided, starting at the posterior border. The retroperitoneal space is exposed and Gerota’s fascia is identified. The 12th rib may need to be resected to allow for better exposure. However, this will increase the risk of a postoperative pneumothorax (0.09%).25 Gerota’s fascia is then incised. The ureter is identified and dissected down to the iliac vessels, at which point it is clipped and divided, preserving an appropriate length for subsequent transplantation. Tissue overlying the renal artery and vein is identified and divided. At this point, the kidney is isolated on its vascular pedicle. When the recipient team is ready, a right angle clamp is placed on the renal artery and the artery is divided. A Satinsky clamp is placed around the inferior vena cava for a right nephrectomy or on the renal vein for a left nephrectomy. The renal vein is divided and the kidney is given to the recipient team. The renal artery stump is then suture-ligated. The renal vein stump is oversewn with 5-0 Prolene sutures in a running fashion.
Postoperative Care and Follow-Up
Postoperatively, the patient should be kept well-hydrated and careful attention paid to urine output. The diet can be advanced quickly in open or laparoscopic cases. The most common complications include urinary retention and ileus. Other less common complications include bleeding, deep venous thrombosis or pulmonary embolus, rhabdomyolysis, and injury to the bowel, bladder, or spleen. Patients who undergo laparoscopic donor nephrectomy tend to have shorter hospital stays (2 to 4 days) compared with patients who have undergone open nephrectomy (3 to 7 days).23
The long-term consequences of kidney donation have been carefully reviewed. However, a long-term donor registry has still not been created. Survival and the development of end-stage kidney disease do not appear to be affected by living donation. In a study of 3698 kidney donors from 1963 to 2007 at a single center, it was shown that end-stage renal disease (ESRD) developed in 180 cases/million persons/year in donors compared with 268 cases/million persons/year in the general population.26 Scores of physical and mental health in this population were significantly better than those of the general U.S. population. In summary, living donation is a safe procedure that does not adversely affect the future health of carefully screened people. Previous living kidney donors enjoy an excellent quality of life and the rate of change in GFR has not been found to exceed that of the general population.
Deceased Donors
As noted earlier, organs are procured from standard criteria or extended-criteria donors. Procurement occurs after declaration of death, either brain death (Box 28-3) or cardiac death. The University of Wisconsin has created a tool to determine the likelihood of progression to cardiac death to allow centers to inform families better (Fig. 28-2).27 A complete neurologic examination must first be completed when the patient has a core temperature above 32° C and there is no evidence of drug intoxication, poisoning, or neuromuscular blocking agents. There can be no other medical conditions that could confound the clinical assessment, such as severe electrolyte, acid-base, or endocrine disturbances or hypotension. A complete clinical neurologic examination includes documentation of coma, the absence of brainstem reflexes, and apnea. Confirmatory testing is also completed, as outlined in Box 28-3.
Box 28-3 From Wijdicks EF: The diagnosis of brain death. N Engl J Med 344:1215–1221, 2001.
Confirmatory Testing Criteria for Determination of Brain Death
Cerebral Angiography
The contrast medium should be injected under high pressure in anterior and posterior circulation.
The external carotid circulation should be patent.
The filling of the superior longitudinal sinus may be delayed.
Electroencephalography
A minimum of eight scalp electrodes should be used.
Interelectrode impedance should be between 100 and 10,000 Ω.
The integrity of the entire recording system should be tested.
The distance between electrodes should be at least 10 cm.
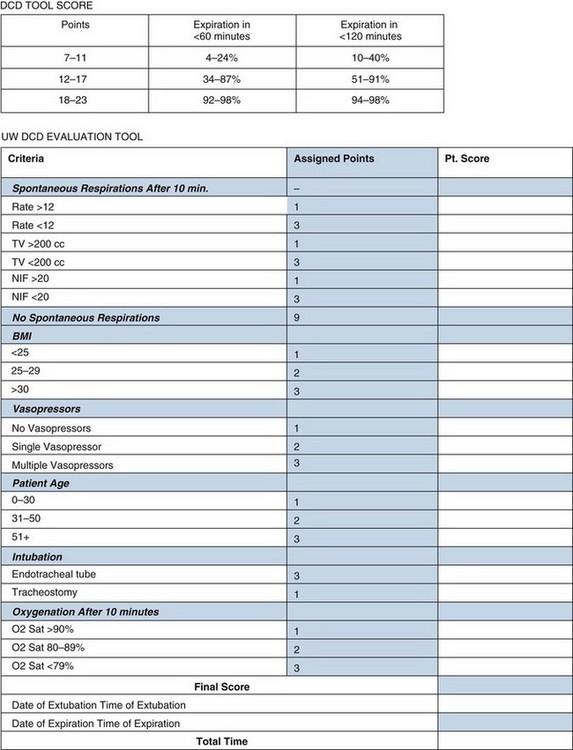
FIGURE 28-2 Tool for predicting progression to cardiac death
(Adapted from Lewis J, Peltier J, Nelson H, et al: Development of the University of Wisconsin Donation After Cardiac Death Evaluation Tool. Prog Transplant 13:265–273, 2003.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


